
Error: No layouts found
On a lab bench in an Einstein laboratory, the tiny one-day-old fish embryo, barely visible to the naked eye, basks and occasionally twitches in the warm water of a petri dish. Move over fruit flies, roundworms, rats and mice: There’s a new animal model in town, and it’s making a big splash at Einstein and in laboratories around the world. It hails from the Ganges River and its name is Danio rerio—more commonly known as the zebrafish.
George Streisinger, a molecular biologist at the University of Oregon as well as a fish hobbyist, began developing the zebrafish as an animal model in the 1970s. Today, thousands of researchers worldwide rely on these tiny swimmers for help in better understanding vertebrate development and human disease. Zebrafish offer key advantages over other model organisms. They’re vertebrates, so they resemble us more closely than do fruit flies or roundworms. They are easily maintained and are prolific breeders, with each female laying 200 to 300 eggs daily. Embryos hatch just three days after fertilization. (Mice, by contrast, have a three-week gestation period and cost much more than zebrafish to feed and house.)
Zebrafish eggs are fertilized outside the mother, making the embryos easily accessible for study. But the real beauty of zebrafish is that their eggs and embryos are transparent, so researchers can watch organs develop in real time. Their embryonic development is remarkably similar to ours, with one big exception: Zebrafish develop much faster.
“In just 24 hours, zebrafish embryos have developed all their organs, including a brain and a heart—equivalent to the first month of human embryonic development,” says zebrafish researcher Florence L. Marlow, Ph.D., an associate professor in the department of developmental and molecular biology and in the Dominick P. Purpura Department of Neuroscience.
About 70 percent of human genes have zebrafish counterparts, and mutation studies involving zebrafish have yielded crucial genetic information. Zebrafish mutations can readily be induced by adding mutagens to aquarium water (traditionally directed at the germ cells of adults) or by using CRISPR/Cas9 (the recently developed gene-editing technique) to cause targeted mutations in embryos.
After mutations are induced in the germline (eggs and sperm), the effects can be evaluated by crossing males and females carrying the same mutation and examining their progeny for changes in appearance, behavior or physiology. Sequencing the genomes of fish exhibiting such phenotypic differences reveals the affected gene. And by knowing the phenotype and that gene’s human counterpart, researchers can find the human gene’s normal function.
Zebrafish mutants exhibiting phenotypes that mimic human diseases have allowed researchers to pinpoint the genetic and cellular defects responsible for a number of diseases, including Duchenne muscular dystrophy and hereditary spastic paraplegia. And as a bonus, testing different drugs on zebrafish disease models can yield treatments for their human counterparts.
Dr. Marlow and two Einstein colleagues (below pages) use zebrafish to study vertebrate development. They primarily study zebrafish larvae, the free-swimming and most advanced embryo stage. Their research may reveal strategies for combating human health problems such as infertility, cancer and congenital spinal disorders.

The cells that give rise to zebrafish eggs might look perfectly symmetrical. But closer scrutiny with a microscope shows that these cells, known as oocytes, are asymmetric on the inside. Scientists first noticed oocyte polarity more than a century ago when they spotted a clump of organelles and proteins on one side of the cell. This structure is the Balbiani body—an aggregate of organelles including endoplasmic reticulum, golgi and mitochondria. It is found in the oocytes of all animals, from fruit flies to fish to humans.
Dr. Marlow has long been fascinated by oogenesis, the process by which an oocyte becomes a fully functional egg and, eventually, an embryo. The oocyte’s polarity seems to play a crucial role in transforming oocytes into eggs and then into embryos with the proper developmental blueprint.
In earlier research, she discovered that a gene called bucky ball assembles the Balbiani body, which is essential for generating polarity in vertebrate oocytes. She observed that zebrafish carrying bucky ball mutations produced oocytes with no polarity and no Balbiani bodies—which proved disastrous. “The mutant mothers produce eggs,” she says, “but those eggs can’t develop into embryos.”
Before their own genomes kick in, the embryos of zebrafish and other vertebrates rely exclusively on maternal gene products (RNAs and proteins) for their early development. These gene products are either made by the developing oocyte or imported into the oocyte (typically via the maternal bloodstream or from nearby follicle somatic cells). Genes that provide essential maternal functions during oogenesis and embryonic development are “maternal-effects genes.” Dr. Marlow had shown that bucky ball is a maternal-effect gene, vital for assembling the Balbiani body and creating oocyte polarity. Did its influence on cell polarity extend to the early zebrafish embryo as well?
To find out, Dr. Marlow identified other proteins that bind to Bucky ball protein. She then looked at where in zebrafish embryos those proteins were produced, and induced gene mutations to assess the functions of these proteins. The Bucky ball−binding proteins turned up in neurons and in cartilage-forming cells in the jaw, where they were found to play important roles. Both cell types share a key trait with oocytes: polarity. Neurons, for example, are visibly asymmetrical, with thick cell bodies and long, skinny protrusions that send and receive information. “They have to be polarized so that they can function,” Dr. Marlow says.
While polarity depends on bucky ball, it also requires trafficking—the process that enables molecules to travel inside a cell. All cells have cellular transport systems, but polarized cells depend especially on trafficking to ensure that proteins, RNA and other cargo arrive and are produced in the right place at the right time.

Clockwise from top left:
In this early zebrafish embryo (blastula stage, approximately four hours after fertilization), cell membranes are stained red and nuclei are blue. A cell in the upper middle part of the image is undergoing mitosis.
In this ventral view of a five-day-old zebrafish larva (head at the top and posterior at the bottom), mitochondria and endoplasmic reticulum are stained green. The cartilaginous lower jaw is outlined by its matrix proteins (purple), which encompass numerous chondrocytes (yellow and green).
Oocytes are the large spheres in this high-magnification image of an adult zebrafish ovary (some nine oocytes are visible, three outlined with white dashes). The oocytes are surrounded by somatic follicle cells (blue nuclei) and blood vessels (yellow).
“When an egg cell can’t traffic goods, you get abnormal embryos,” Dr. Marlow says. In neurons, “you get neurodegenerative diseases. And aberrant trafficking in cartilage-forming jaw cells causes abnormal growth and malformation of the head and facial bones.”
Dr. Marlow has collaborated with Einstein’s Robert H. Singer, Ph.D., to develop a technique to observe trafficking in a zebrafish embryo. (Dr. Singer is professor, co-chair and the Harold and Muriel Block Chair of anatomy and structural biology, a professor of cell biology and of neuroscience, and co-director of Einstein’s Gruss Lipper Biophotonics Center and of its EGL Charitable Foundation Integrated Imaging Program.)
She brings up a movie of a zebrafish neuron on her computer. A bright green dot travels from the right side of the screen to the left. That dot is messenger RNA tagged with a fluorescent protein, and it’s traveling from the neuron nucleus through the cytoplasm en route to a nearby synapse. “Nobody has seen this in a live vertebrate animal before,” she notes.
Using this imaging technique, Dr. Marlow hopes to better understand the factors that regulate trafficking. Improved understanding of how this process works in fish—and what happens when it doesn’t—may provide insights into infertility as well as neurodegenerative diseases such as Alzheimer’s, Parkinson’s and Huntington’s diseases and hereditary spastic paraplegia.

After they’re transcribed from genes but before being translated into proteins, many vertebrate messenger RNA molecules (mRNAs) undergo splicing: noncoding mRNA regions (introns) are removed, and the flanking coding regions (exons) are bound together. This mRNA splicing and binding is carried out by spliceosomes—giant, complex molecular machines found in the nucleus and composed of small nuclear RNAs and protein complexes. Spliceosomes help regulate gene expression and increase mRNA diversity. And for unknown reasons, they can cause cancer when defective. Zebrafish may reveal why.
About four years ago, researchers studying patients with myelodysplastic syndrome (MDS)—often a precursor to acute myeloid leukemia (AML)—noticed that MDS patients often had mutations affecting their spliceosome components. “This was quite unexpected—no one had thought that spliceosome mutations could potentially lead to cancer,” says Teresa V. Bowman, Ph.D., an assistant professor of developmental and molecular biology and of medicine (oncology) and a member of the Ruth L. and David S. Gottesman Institute for Stem Cell and Regenerative Medicine Research at Einstein. “Many labs are now trying to figure out why—and ours is too, using zebrafish as our in vivo model system.”
Spliceosomes contain many proteins encoded by different genes. Dr. Bowman and her team have focused on one spliceosome gene in particular, called SF3B1. It is the most frequently mutated gene in MDS, affecting as many as 60 percent of MDS patients. Single-point mutations in an SF3B1 allele somehow lead to defective hematopoietic (blood-forming) stem cells, which in turn results in anemia, neutropenia and the other blood abnormalities that characterize MDS.
“Because of where they’re located in the gene and its corresponding protein, it’s unclear how the mutations are changing the protein’s function,” says Dr. Bowman. “Zebrafish have that same gene, and we’re studying how a somewhat different sf3b1 mutation—a loss-of-function mutation—affects the blood system in zebrafish embryos, whose blood systems fully develop in just 24 hours. What we find may lead to strategies for correcting the hematopoietic problems that afflict MDS patients.”
While the mutant zebrafish embryos and MDS patients don’t have precisely the same mutations in that gene, they do exhibit some of the same blood abnormalities, including anemia and neutropenia (an abnormally low number of neutrophils). “We’ve observed striking similarities in the blood-cell defects and gene-expression changes that occur in the hematopoietic cells of MDS patients and in our zebrafish mutants,” says Dr. Bowman. “Knowing this, we can use the extensive amount of genetic information produced by zebrafish research to help us figure out what’s going on in MDS.”

An image from Dr. Bowman’s lab of an early zebrafish embryo (36 hours postfertilization). Blood vessels are stained red and blood cells are stained green.
Meanwhile, Dr. Bowman is searching for new drugs that could reverse the blood defects that her zebrafish embryos share with MDS patients. “We’re testing more than 700 compounds in the hope of finding a new molecule that may be able to treat the abnormal phenotype caused by the SF3B1 mutation—either by restoring normal function to the mutant cells or by preferentially killing those cells while sparing the nonmutated, healthy cells,” says Dr. Bowman.
By inhibiting an aberrant signaling pathway in MDS, for example, a compound might be able to “rescue” abnormal hematopoietic stem cells, enabling them to make more red blood cells and thereby reversing anemia. Her lab has already found that the anti-inflammatory steroid dexamethasone partially rescues hematopoietic stem-cell function in mutant zebrafish. And since MDS is often a precursor to AML, “improving the function of MDS patients’ hematopoietic stem cells could lead to greater numbers of healthy white and red blood cells and potentially prevent patients with MDS from developing AML,” Dr. Bowman notes.


A zebrafish embryo tail imaged with fluorescent microscopy in Dr. Ozbudak’s lab. Blue circles are nuclei; red dots are single RNA molecules transcribed from the segmentation clock gene (her7). The oscillatory expression of the her7 gene generates traveling waves in cells located in the presomitic mesoderm (U-shaped tissue containing the precursors of vertebrae). The oscillation pulses start at the tail tip (left), generate waves (stripes) as they travel through the tissue and finally halt at the end of the tissue (right).
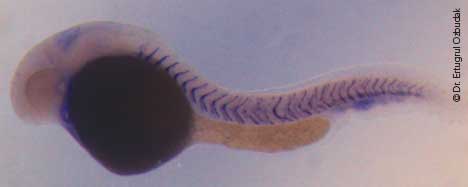
This zebrafish larva is a her7 homozygous mutant, resulting in defective somites (the precursors of vertebrae). The zebrafish her7 gene corresponds to the human Hes7 gene, which when mutated causes scoliosis. The purple chevron-shaped lines mark the boundaries between somites. Here, the first 10 somites closest to the zebrafish head appear normal, but more-posterior segments have broken boundaries. The yolk sac is the large dark mass that extends beneath the somites.
Ertugrul M. Ozbudak, Ph.D., an associate professor in the department of genetics, studies how backbones develop—the process known as vertebral segmentation. His group and other researchers have identified several genes that play important roles in that process. But some key ones remain to be found.
In all vertebrates, whether zebrafish or zebras, the backbone begins as a clump of cells. This clump must be segmented so that individual vertebrae can form. The segmentation of these cells into vertebral precursors called somites happens at a precise tempo. “As the embryo elongates, try to visualize a pendulum swinging back and forth and slicing the clump of cells into the individual somite segments,” Dr. Ozbudak says.
The timing must be exact. In human embryos, one segment forms every five hours. In zebrafish embryos, the clock ticks faster: a somite forms every half hour. The process relies on oscillating genes—with each swing of the pendulum, expression of some genes rises and for others it falls. Dr. Ozbudak and colleagues have found evidence that zebrafish and other vertebrates use a similar “segmentation clock”—a gene-expression oscillator that paces the rhythmic segmentation of the vertebral column during embryonic development.
In a room jammed with dozens of fish tanks, Dr. Ozbudak searches for one particular kind of fish. It has a kinked spine, the result of a genetic mutation (see image above). Mutations in genes controlling the oscillator mechanisms are suspected of causing most of the spinal defects afflicting zebrafish as well as people.
Many oscillator-mechanism genes have been identified. But the pacemaker—the crucial gene (or, more likely, several coordinated genes) controlling how fast the pendulum swings—remains elusive. Dr. Ozbudak is in hot pursuit, his search fueled in part by a recent five-year, $1.9 million grant from the National Institutes of Health. One fact helps narrow the list of pacemaker gene suspects: For oscillation to work properly, the pacemaker protein that propels each swing of the pendulum must be short-lived.
Dr. Ozbudak, who trained as a physicist before becoming a biologist, constructed a mathematical model to estimate how short-lived the pacemaker protein must be for the system to work. While the average protein lasts nearly two days, his model predicted that the pacemaker protein should have a much shorter half-life: six minutes or less.
He and his colleagues have zeroed in on one particular oscillator-mechanism gene, called her7, that appears to repress the expression of other oscillator-mechanism genes in zebrafish embryos. The her7 gene’s protein product, Her7, has a half-life of just three and a half minutes. This, says Dr. Ozbudak, makes her7 a strong candidate to at least be a component of the segmentation clock’s pacemaker. Moreover, her7 has a human counterpart—Hes7—that causes congenital scoliosis when mutated.
Dr. Ozbudak’s lab is now working to identify the genes that regulate Her7’s rapid turnover in zebrafish. Finding those genes should provide a clearer picture of just what makes the segmentation clock tick—and may help reveal the gene defects responsible for congenital spinal disorders.