
Error: No layouts found
The ideal way to study disease at the molecular level is to analyze cells from the affected tissues of patients—not a problem for, say, dermatologists or hematologists, who can readily obtain skin or blood cells. But neuroscientists lack such access. The brain, encased in its bony vault, is well protected from insult, injury and prying hands. So those who study brain diseases have had to make do with tissue samples obtained at autopsy.
“This has been extremely limiting,” says Herbert Lachman, M.D., professor of psychiatry and behavioral sciences and of medicine (hematology). “Diseases such as schizophrenia may begin as early as embryogenesis. But with autopsy specimens, you’re typically looking at cells from adults, many decades after the disease first developed. In addition, the cells may be from someone who abused drugs or alcohol or had taken psychotropic medications, which can make it difficult to distinguish the primary disease from secondary influences.”
Dr. Lachman has embraced iPSC technology because he realizes it could provide him with live nerve cells (neurons) from living patients.
“It was a steep learning curve—iPSC technology is extremely complex, and I made a lot of rookie mistakes,” he admits. But his efforts are paying off. Three years down the line, he has mastered the fine art of transforming skin cells into iPSCs and then tweaking iPSCs into neurons, creating a bounty of research opportunities.
In a study funded by the National Institute of Mental Health, Dr. Lachman is comparing iPSC-derived neurons from patients with schizophrenia to neurons from healthy controls. He’s particularly interested in whether neurons from the two groups differ in their microRNAs—snippets of RNA that regulate gene expression.
MicroRNAs are known to play a key role in brain development and in forming synapses (connections between neurons), says Dr. Lachman, who is also an associate professor in the Dominick P. Purpura Department of Neuroscience and the department of genetics. And evidence from a genetic disease called velo-cardio-facial syndrome (VCFS) points to a role for microRNAs in schizophrenia.
VCFS is caused by a 22q11 microdeletion (the absence of a small portion of chromosome 22). Approximately one-third of VCFS patients suffer from schizophrenia. The specific gene defect responsible for these cases of VCFS has not been unequivocally identified, but one promising candidate is DGCR8, which codes for a protein involved in microRNA production.
Mice genetically engineered to have just one copy of DGCR8, instead of the usual two, exhibit changes in behavior, in neuronal branching (a measure of brain-cell connectivity) and in the expression of microRNAs in the hippocampus and cortex.
“Our goal is to find out which microRNAs are abnormally regulated in schizophrenia,” says Dr. Lachman, also an attending physician at Montefiore. In theory, those microRNAs could then be targeted with medications.
Dr. Lachman also wants to know whether microRNA expression is altered in patients with schizophrenia who do not have a 22q11.2 deletion. “If so, this would suggest that a single aberrant molecular pathway could account for the problems that characterize this disease, even if schizophrenia itself can originate from many different gene abnormalities,” says Dr. Lachman. “That would certainly simplify the search for a single treatment that would help most people with schizophrenia.”
In other iPSC research, Dr. Lachman is employing iPSCs to grow “mini-brains” in laboratory culture. No Frankenstein worries here: These creations are not brains in the traditional sense but rather small, in vitro three-dimensional aggregates of radial glial cells (neuron precursors) and maturing neurons. The mini-brains are intended to mimic neuronal structures that form in the developing forebrain.
“From autopsy samples, we know that a good fraction of patients with schizophrenia have abnormalities in their synaptic architecture,” he says. “Using these models, we can start looking at this architecture and see how it might be influenced by 22q11 deletions.” He notes that mini-brains could also be used to evaluate new medications for schizophrenia and other diseases.
Our goal is to find out which microRNAs are abnormally regulated in schizophrenia.
A third iPS cell project involves work that Dr. Lachman is doing with Brett S. Abrahams, Ph.D., assistant professor of genetics, to study neuron abnormalities in autism spectrum disorders (ASD).
As in his schizophrenia research, he is using iPSC technology to derive neurons from healthy children and compare them with neurons from children with ASD. The researchers are looking for variations in a portion of chromosome 15 known as 15q11.2. Deletions and duplications within this small area can increase the risk for autism and other behavioral disorders. Some individuals with these variations have no neurodevelopmental issues, while others are severely affected.
By looking at the molecular differences between neurons of affected individuals and healthy controls, Drs. Lachman and Abrahams hope to find precisely how variations at 15q11.2 increase the risk for autism and, ultimately, to develop ways of counteracting the effects of those gene defects.
Despite the great potential for creating disease models using iPSCs, Dr. Lachman cautions that it will take some time before therapies based on this technology reach patients. “Research into gene therapy began several decades ago, and we’re just starting to see results,” he says. “The same will probably hold true for iPSCs.”
Dr. Frenette is similarly cautious: “We don’t know whether iPSCs are exactly the same as stem cells that form naturally. Another issue is that the specialized cells derived from iPSCs have not always progressed to full maturity. Plus, when you have iPSCs that have not fully differentiated, there’s the small but real risk that they could conceivably cause cancer. In other words, we still have a lot of work to do.”
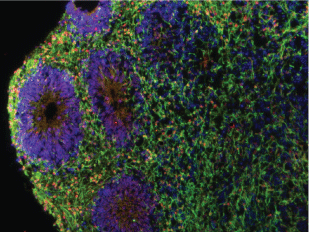
Top of page, cross-section of an aggregate of neurons, referred to as a “minibrain.” It originated from skin fibroblasts of a patient with a chromosome 22q11.2 deletion and schizophrenia. The fibroblasts were reprogrammed into iPSCs that closely resemble human embryonic stem cells.
Dr. Herb Lachman and lab technician Erika Pedrosa coaxed the iPSCs to develop into the “minibrain,” which models early brain development. Embedded in the sea of neurons (stained green) are circular structures resembling neural tubes (stained blue) that consist of radial glial cells organized around a central lumen. A protein encoded by a 22q11.2-linked gene (RANBP1) is stained orange.
Image credit: Erika Pedrosa, M.S.