Inflammation is a condition in which tissues become reddened, swollen, hot, and often painful. It’s what happens when the immune system defends the body against some sort of threat—when you develop a viral or bacterial infection, for example, or burn your hand. In fact, the immune system’s arsenal includes pro-inflammatory signaling proteins such as cytokines, which encourage tissue inflammation as a way to trap and kill microbes and encourage the healing process.
But sometimes, even after a threat has disappeared, the immune response persists—and the resulting chronic inflammation can be devastating, especially to the brain.
Alzheimer’s disease, Parkinson’s disease, multiple sclerosis, Huntington’s disease: research in recent years has shown that all these neurodegenerative brain diseases are fueled by chronic inflammation. Here we describe the work of several Einstein scientists who are studying different aspects of neuroinflammation with the goal of taming it to treat disease and perhaps preventing it from occurring.
For an object no bigger than an almond, the brain’s hypothalamus plays an outsized role in regulating homeostasis—the coordination and regulation of many vital physiological processes so that they interact to maintain a stable, healthy state. Those processes include growth, development, body temperature, blood pressure, sleep, and energy balance (hunger and satiety).
Stress placed on the hypothalamus can cause damaging inflammation that disrupts its grip on homeostasis and leads to metabolic problems. A key cause of that stress is nutrient overload—chronic overeating or consuming a high-fat diet. Dongsheng Cai, M.D., Ph.D., professor of molecular pharmacology, director of Einstein’s Institute for Neuroimmunology and Inflammation, and the Young Men’s Division Chair in Physiology, was the first researcher to show that hypothalamic inflammation due to overnutrition is a root cause of obesity and type 2 diabetes.
Overnutrition creates an abnormally rich mix of molecules in the peripheral bloodstream: not only high levels of saturated fats and glucose but also cytokines and other pro-inflammatory molecules released by the immune system in response to chronic nutrient overload. After crossing the blood-brain barrier (BBB) and becoming absorbed by brain tissue, these overnutrition-related molecules activate the NF-kB inflammatory signaling pathway inside cells of the hypothalamus.
“We know that even low-grade, chronic inflammation of hypothalamic neurons can lead to major metabolic problems, including obesity and diabetes as well as hypertension,” says Dr. Cai, who coined the term “hypothalamic microinflammation” to describe the physiological cause of those health problems and whose research is supported by the National Institutes of Health (NIH).
Even low-grade, chronic inflammation can lead to major metabolic problems, including obesity and diabetes.
— Dr. Dongsheng Cai
Human studies have increasingly linked low-grade inflammation of the body with aging—“inflammaging,” for short. Dr. Cai suspected that inflammation of the hypothalamus might influence not only metabolic issues but also aging.
In a paper published in 2013 in Nature, Dr. Cai showed that—independent of diet—the NF-kB inflammatory pathway becomes active in hypothalamic cells when mice reach middle age and becomes increasingly active as the mice grow older; he and his colleagues were able to extend the life span of mice by suppressing the NF-kB pathway in the hypothalamus.
The researchers then determined how hypothalamic inflammation induces aging: by specifically destroying stem cells in the hypothalamus that replenish neurons. In that study, published in 2017 in Nature, he and his colleagues generated mouse hypothalamic stem cells that were resistant to inflammation. The researchers showed that injecting the inflammation-resistant stem cells into the hypothalamus of middle-aged mice dramatically extended their life spans.
These findings indicate that inflammation of the hypothalamus plays a key role in aging and in controlling the life span. Moreover, suppressing or preventing hypothalamic inflammation might offer a strategy for combating age-related health problems and extending our lives.
When obesity- and aging-related substances in the bloodstream find their way to the hypothalamus, they appear to first enter astrocytes—cells that surround, support, and nourish hypothalamic neurons. Dr. Cai has shown that sustained exposure to these substances activates NF-kB inflammatory pathways in astrocytes of mice; eventually, this activation spreads from astrocytes to various inflammatory pathways in nearby neurons and to other cells of the hypothalamus.
In ongoing NIH-funded research, Dr. Cai is investigating how astrocytes are altered in response to pro-inflammatory signaling and how they “communicate” that inflammatory signaling to neurons—leading to metabolic dysregulation and, ultimately, to obesity and diabetes.
For another NIH-funded study, Dr. Cai is investigating how the link between astrocytes and neurons in the hypothalamus influences the development of obesity-related hypertension, which is responsible for about three-fourths of all cases of high blood pressure.
What causes the behavior changes that occur in people who become depressed? Growing evidence suggests that the answer is inflammation—the body’s response to harmful stimuli. Einstein scientists Joan W. Berman, Ph.D., and Anjali Sharma, M.D., M.S., are multi–principal investigators participating in NIH-funded studies looking at neuroinflammation’s role in causing depression in people with HIV (PWH). Vilma Gabbay, M.D., now an adjunct professor at Einstein, is also a multi–principal investigator on the two NIH grants discussed directly below.
Depression is the most common neuropsychiatric illness among PWH and is estimated to affect nearly 40% of them. By 2030, the top two leading causes of disease burden globally are predicted to be HIV and depressive disorders. Despite these alarming statistics, there has been little research on the molecular mechanisms by which HIV infection may be causing depression.
Drs. Berman and Sharma are seeking the neurobiological mechanisms that may connect HIV infection with depression. Their study is enrolling 300 male and female participants—depressed PWH, nondepressed PWH, depressed HIV-negative people, and healthy controls—and it involves experts in psychiatry, neuroimaging, HIV, and immunology.
Dr. Berman is a professor of pathology and of microbiology & immunology and the Irving D. Karpas Chair in Medicine at Einstein, and co-director of the Biomarkers and Advanced Technologies Core of the Einstein-Rockefeller-CUNY Center for AIDS Research (CFAR); Dr. Sharma is a professor of medicine at Einstein and an internist and infectious-disease physician at Montefiore, and co-chairs the HIV and Mental Health Scientific Working Group at CFAR.
The researchers hypothesize that systemic inflammation disrupts the blood-brain barrier, allowing HIV-infected blood cells called monocytes to cross the BBB and then release cytokines that inflame and alter the brain’s reward circuitry and contribute to depression. If the researchers can confirm this chain of events, they may be able to develop therapies to ward off depression in PWH.
Dr. Sharma is a multi–principal investigator on a study involving PWH, with a focus on women living with HIV (WLWH). Such women are greatly affected by depression and yet are underrepresented in HIV research. Dr. Sharma and her colleagues hypothesize that WLWH are especially susceptible to neuroinflammation that affects the brain’s reward circuits. Like the study of PWH, this study is enrolling 300 participants. Sixty percent of them are women.
The researchers believe that two key adverse events occur when the central nervous system becomes inflamed by HIV: chemicals called free radicals injure nerves of the central nervous system, and levels of gamma-aminobutyric acid—a neurotransmitter known to have a calming effect—are reduced. These neurochemical changes are believed to be involved in depression. The findings from this research may lead to strategies for improving mental health as well as overall health in PWH.
Antiretroviral drugs have saved the lives of countless people with HIV. Yet these drugs can’t completely eradicate the virus, leaving potentially dangerous reservoirs of HIV in various parts of the body, including the brain.
“These HIV reservoirs may not be actively replicating, but they’re still shedding viral proteins,” Dr. Berman explains. “That’s a particular problem in the brain, where the proteins are both toxic and inflammatory.”
Dr. Berman believes that HIV protein shedding is partly responsible for neuroAIDS—a neuroinflammatory disorder that affects as many as 40% of people with HIV and involves cognitive impairments ranging from mild to severe (although, she says, dementia is less common in the era of antiretroviral drugs). Her research focuses on the blood-brain barrier—a tightly packed, semipermeable layer of endothelial cells that forms the inner surface of blood vessels inside the brain. She studies how alterations in its functions contribute to neuroinflammation and neuroAIDS.
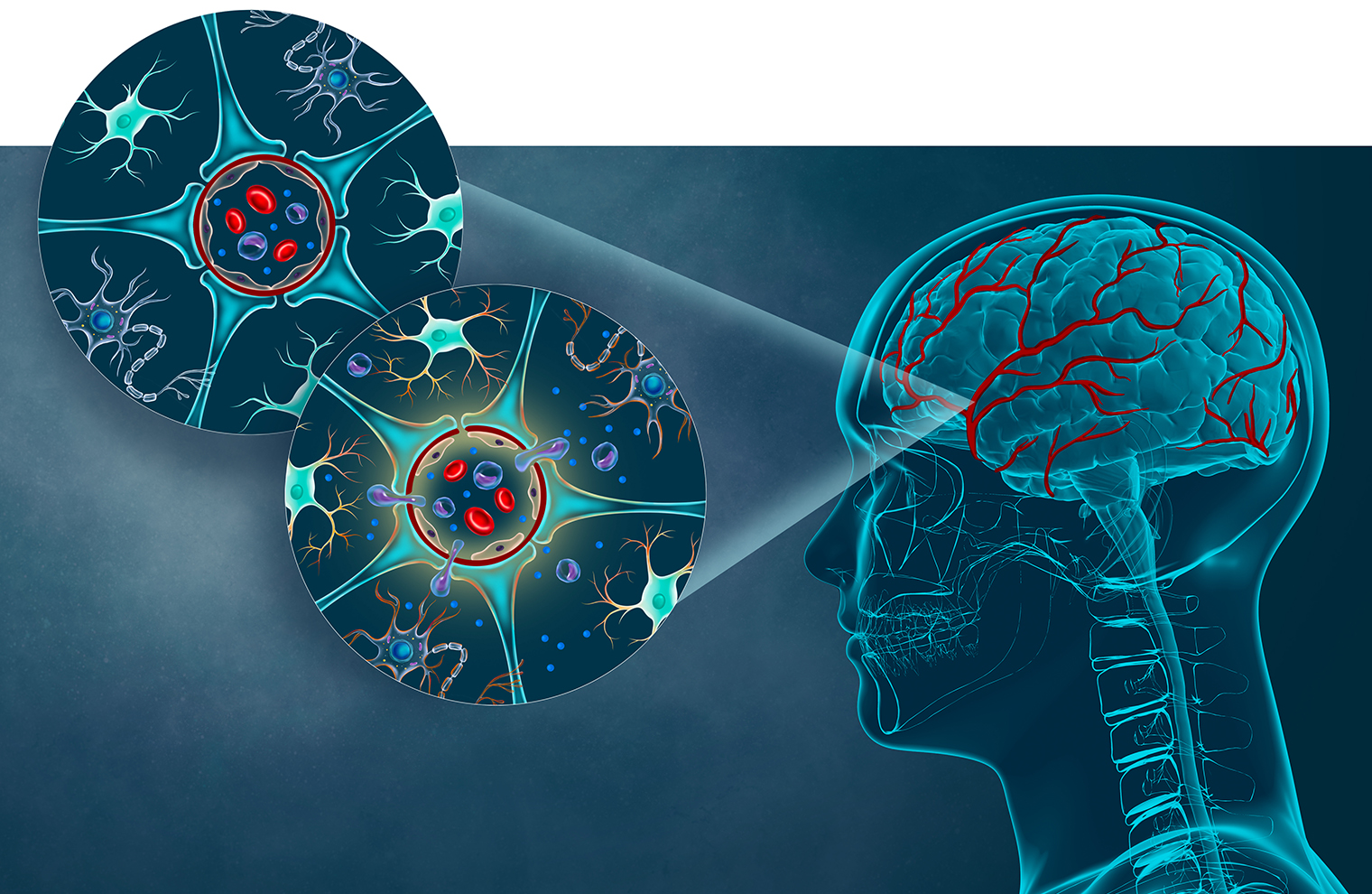 The blood vessels that form the blood-brain barrier (BBB, shown at right) possess cells with unique properties. The upper-left cross section shows that the BBB consists mainly of specialized, closely connected endothelial cells (forming a red ring) plus astrocytes (blue) that connect to endothelial cells with their end feet. The two cell types work together to regulate the movement of molecules, ions, and cells between the blood and the brain. The serum of people with HIV may contain inflammatory proteins that damage the BBB (cross section at lower left), allowing HIV-infected monocytes to cross into brain tissue and contribute to neuroAIDS. (Illustration by Tatyana Harris)
The blood vessels that form the blood-brain barrier (BBB, shown at right) possess cells with unique properties. The upper-left cross section shows that the BBB consists mainly of specialized, closely connected endothelial cells (forming a red ring) plus astrocytes (blue) that connect to endothelial cells with their end feet. The two cell types work together to regulate the movement of molecules, ions, and cells between the blood and the brain. The serum of people with HIV may contain inflammatory proteins that damage the BBB (cross section at lower left), allowing HIV-infected monocytes to cross into brain tissue and contribute to neuroAIDS. (Illustration by Tatyana Harris)
The BBB normally keeps microbes and potentially harmful cells and other substances from reaching the brain, while letting through certain cells as well as water, oxygen, nutrients, and general anesthetics. Early in the course of HIV infections, however, HIV-infected immune cells called monocytes are able to cross the BBB, leading to the inflammatory damage to brain tissue that results in neuroAIDS. (See illustration above.)
About two decades ago, soon after starting her study of neuroAIDS, Dr. Berman developed a tissue-culture model of the human BBB. That model, which she has refined over the years, is now used by researchers in several dozen laboratories worldwide. In research funded by NIH grants, Dr. Berman is working with her BBB model to learn how HIV-infected monocytes manage to cross the BBB and precipitate inflammation—and how to halt that journey to prevent neuroAIDS.
Ideally, the BBB should prevent HIV-infected monocytes from entering the brain while not impeding uninfected monocytes that the brain needs for maintaining immune surveillance. One of Dr. Berman’s research challenges is determining how the two types of monocytes differ.
“We’re looking for cell-surface proteins that are found only on HIV-infected monocytes and that may facilitate passage of infected cells across the BBB,” says the researcher. “If we could selectively target those proteins with drugs or other therapeutic interventions, we might be able to slow the entry of infected monocytes into the brain. Similarly, we might be able to target inflammatory mediators in the serum of people with HIV that damage the BBB and help the infected monocytes cross into the brain.”
Dr. Berman also has NIH grants to study people with substance-use disorders who have HIV—a group especially prone to cognitive impairment. Her studies show that both opioids and methamphetamine may worsen neuroAIDS by making the BBB more permeable, enabling HIV-infected monocytes to cross the BBB and cause tissue-damaging inflammation in the brain. Studies in her lab, and by her collaborators at the Icahn School of Medicine at Mount Sinai, suggest a possible therapy for the inflammation that fuels neuroAIDS: buprenorphine, a drug used to treat opioid-use disorder.
“We’ve found that buprenorphine is effective at reducing neuroinflammation,” she explains. “We’d like to test whether it can reduce inflammation in people with HIV, regardless of whether they have substance-use disorders.”
It turns out that brain inflammation isn’t always bad. Do you remember the day you graduated from college? What you did on your last birthday? You can thank inflammatory signaling in certain brain neurons, says Jelena Radulovic, M.D., Ph.D., professor in the Dominick P. Purpura Department of Neuroscience, professor of psychiatry and behavioral sciences, the Sylvia and Robert S. Olnick Chair in Neuroscience, and co-director of the Psychiatry Research Institute at Montefiore Einstein.
Dr. Radulovic uses mice to study various aspects of the neurobiology of memory. Recently she has been investigating how neuroinflammation leads to episodic memories—long-term memories of personal experiences.
Neural circuits connecting two brain regions—the retrosplenial cortex and the hippocampus—are especially important for episodic memory. Dr. Radulovic is studying how inflammatory proteins (those involved in the NF-kB pathway as well as interferon and transforming growth factor beta) form memories in those circuits and enable them to persist. She is also investigating the cell-specific roles played by inflammation in allowing us to access memories throughout our lives.