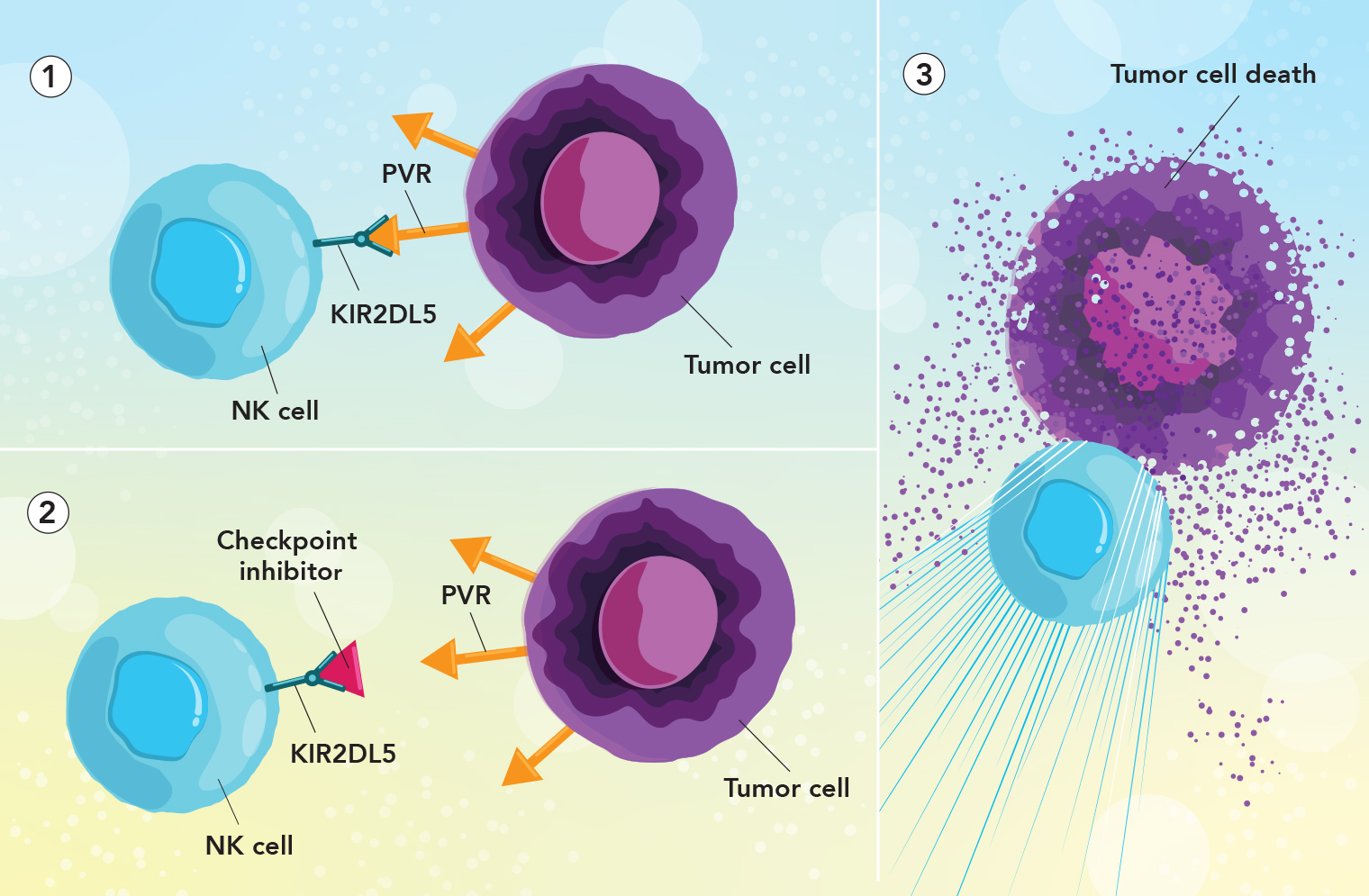
 Freeing up immune cells to attack tumors: 1. The tumor protein PVR (orange arrow) has “turned off” a natural killer (NK) cell by binding with its checkpoint receptor protein KIR2DL5 (green). 2. The new checkpoint inhibitor (red triangle) has blocked the NK cell’s KIR2DL5 protein, preventing PVR from inactivating the NK cell. 3. With the checkpoint inhibitor protecting the NK cell from PVR, the NK cell can now attack the tumor cell.
Freeing up immune cells to attack tumors: 1. The tumor protein PVR (orange arrow) has “turned off” a natural killer (NK) cell by binding with its checkpoint receptor protein KIR2DL5 (green). 2. The new checkpoint inhibitor (red triangle) has blocked the NK cell’s KIR2DL5 protein, preventing PVR from inactivating the NK cell. 3. With the checkpoint inhibitor protecting the NK cell from PVR, the NK cell can now attack the tumor cell.
Immune-checkpoint inhibitors, such as the name-brand drugs Keytruda and Opdivo, unleash the immune system’s T cells to attack tumor cells. Their introduction a decade ago marked a major advance in cancer therapy, but only 10% to 30% of treated patients experience long-term improvement.
In a paper published in November 2022 in the Journal of Clinical Investigation, Xingxing Zang, M.Med., Ph.D., and colleagues presented findings that could bolster the effectiveness of immune-checkpoint therapy. Dr. Zang is the Louis Goldstein Swan Chair in Women’s Cancer Research and a professor of microbiology & immunology, of oncology, of medicine, and of urology at Einstein and a member of the Cancer Therapeutics Program at Montefiore Einstein Cancer Center.
The surfaces of immune cells are studded with receptors called “checkpoint” proteins. By recognizing the body’s “self” proteins, checkpoint receptors prevent T cells from attacking the body’s normal cells. Most types of cancer cells cunningly express “self-like” proteins that can bind with checkpoint proteins, tricking immune cells into standing down and not attacking tumors.
Current checkpoint inhibitors are monoclonal antibodies designed to short-circuit immune-cell/cancer-cell interactions by blocking either the tumor proteins or the T-cell receptors that bind with tumor proteins. Their limited effectiveness prompted Dr. Zang and other scientists to look at checkpoint pathways involving natural killer (NK) cells, which—like T cells—play major roles in eliminating unwanted cells. A cancer-cell protein called PVR soon captured their attention.
PVR protein is usually absent from or very scarce in normal tissues, but is found in abundance on many types of tumors. Moreover, PVRs appeared to inhibit T-cell and NK-cell activity by binding to a checkpoint protein called TIGIT—prompting efforts to interrupt the TIGIT/PVR pathway by using monoclonal antibodies made against TIGIT. However, several clinical studies, including two large phase 3 clinical trials, have recently failed to improve cancer outcomes.
Meanwhile, the cancer-cell protein PVR was found to have another “binding partner” on NK cells: the receptor protein KIR2DL5. “We hypothesized that PVR suppresses NK-cell activity not by binding with TIGIT but by binding with the recently recognized KIR2DL5,” says Dr. Zang. To find out, he and his colleagues synthesized a monoclonal antibody targeting KIR2DL5.
In studies involving humanized animal models of several types of human cancers, the researchers showed that their monoclonal antibody against KIR2DL5—by blocking the KIR2DL5/PVR pathway—allowed NK cells to vigorously attack and shrink human tumors and prolong animal survival.