
Error: No layouts found





The British biologist Sir Peter Medawar described a virus as “a piece of bad news wrapped in protein.” There’s surely not much good to say about these strange, neither-living-nor-dead particles, which multiply with abandon after infecting cells and often sicken or kill their hosts, be they bacteria, plants or mammals. Throughout history, viruses have profoundly affected human health—the smallpox epidemic of the 1600s devastated Native Americans, the “Spanish flu” of 1918 sickened or killed one-third of the world’s population, the HIV epidemic of the late 20th century killed some 35 million. Today, HIV and many other viruses still exact a deadly toll.
Einstein has long maintained research programs aimed at developing vaccines, treatments and cures for viral diseases, with emphasis on viruses that affect people in the developing world. This article describes the latest Einstein research into herpes simplex, chikungunya, Ebola and dengue, which together affect hundreds of millions of people worldwide.
Few viruses provoke more dread than Ebola, which causes Ebola hemorrhagic fever. The first documented outbreak occurred in 1976 and affected villages along the Ebola River in Africa—hence the virus’s name. Three of the five known Ebola virus strains (Zaire, Sudan and Bundibugyo) have caused major outbreaks in humans, and the other two (Reston and Tai Forest) cause severe disease in nonhuman primates. During the 2013–16 West African Ebola outbreak, hemorrhagic fever caused by Ebola Zaire killed 11,000 people and gravely sickened another 29,000.
The Holy Grail of Ebola research is a treatment for all three strains that affect humans. “It’s impossible to predict which of the strains will cause the next epidemic, so we need to develop a single therapy that could treat or prevent infection caused by any known Ebola virus,” says Kartik Chandran, Ph.D., professor of microbiology & immunology and the Harold and Muriel Block Faculty Scholar in Virology, who leads Einstein’s Ebola research effort.
In two papers over eight months, Dr. Chandran, his Einstein colleague associate professor of biochemistry Jonathan Lai, Ph.D., and other researchers described two quite different approaches for developing a single therapy against all Ebola virus strains.
After analyzing the blood of a survivor of the 2013–16 Ebola outbreak, scientists from academia, industry and the U.S. government discovered naturally occurring human antibodies that can neutralize and protect animals against all three Ebola virus strains. The findings, published last May in Cell, could lead to broadly effective Ebola virus therapies and vaccines.
Other members of the research team had previously isolated 349 distinct antibodies from a survivor of the 2013–16 Ebola epidemic. Two of those 349 antibodies, known as ADI-15878 and ADI-15742, were found to kill all five known Ebola virus strains in tissue culture. When combined, the two antibodies also protected mice and ferrets that had been exposed to lethal doses of the strains that cause human disease.
The findings described in the Cell paper showed that the two antibodies bind to the surface of Ebola viruses—more specifically, to a section of a key glycoprotein molecule (a protein with a sugar attached to it). These surface-bound antibodies interfere with the virus’s ability to infect cells and multiply inside them. The researchers learned precisely where on the glycoproteins the antibodies attach and how the antibodies neutralize Ebola viruses—knowledge that should help in crafting broadly effective immunotherapies.
In the September 2016 issue of Science, Drs. Chandran, Lai and colleagues described a different antibody strategy. The researchers developed a novel amalgam of antibodies that blocked all five Ebola virus strains from invading human cells in laboratory studies. More importantly, the antibodies protected mice exposed to lethal doses of Ebola Zaire and Ebola Sudan, the two most dangerous strains.
To gain entry to cells, filoviruses (the family that includes all Ebola viruses, along with the extremely dangerous Marburg virus) use their surface glycoproteins to bind to the host cell’s outer membrane.
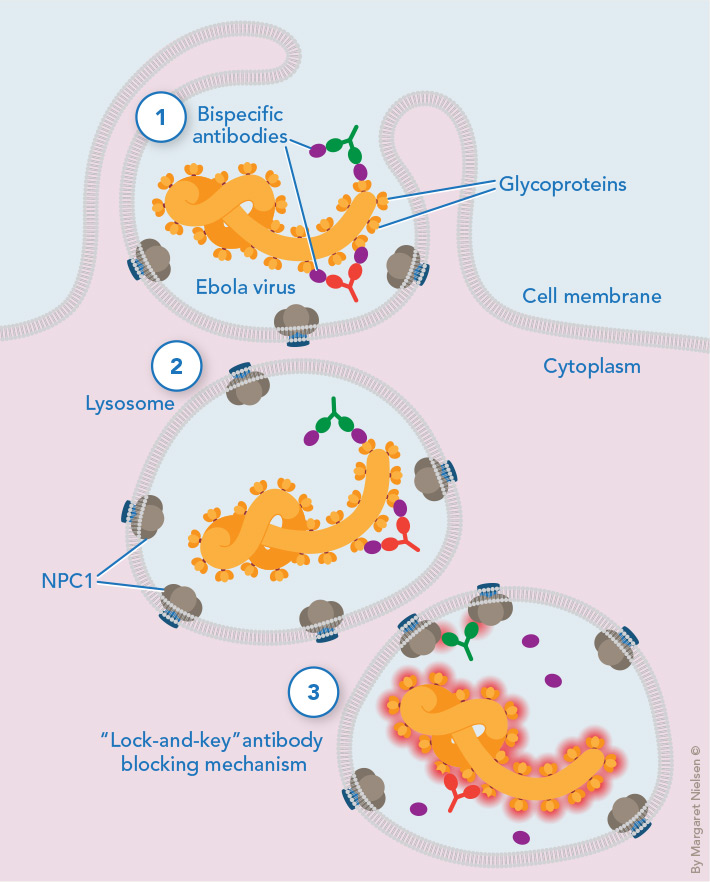
Tricking a deadly virus: Antibodies attached to glycoproteins accompany the Ebola virus when a portion of host cell membrane surrounds the virus and pinches off, allowing the virus to infect the cell (1). The pinched-off region develops into a lysosome, from which the virus must escape so it can multiply in the cytoplasm (2). Escape requires a previously hidden viral protein “key” that fits into a protein “lock,” called NPC1, embedded in the lysosome membrane. Viral escape is thwarted when one antibody (green) blocks the NPC1 “lock,” while the other antibody (red) blocks the virus’s protein key (3).
A portion of this membrane then surrounds the virus and pinches off, eventually developing into a lysosome—a small intracellular bag filled with enzymes that digest foreign and cellular components.
The viruses must break out of their lysosomal “prisons” so they can multiply, and here they get help from the lysosome itself. Lysosomal enzymes slice a “cap” from the virus’s glycoproteins, unveiling a hidden viral protein. This viral protein “key” fits into a “lock”—a protein embedded in the lysosome membrane called Niemann-Pick C1 (NPC1). The fitting of key into lock fuses the filovirus to the lysosomal membrane. Now the virus can propel its RNA genetic material through the lysosome and into the cell’s cytoplasm, where the virus can replicate itself.
Since all filoviruses depend on this lock-and-key mechanism to reproduce, the researchers recognized this as a filovirus Achilles’ heel they could exploit using synthetic monoclonal antibodies (identical antibodies produced by a single clone of cells). They obtained one monoclonal antibody to block the viral key and another (developed at Einstein by Matthew Scharff, M.D., distinguished professor of cell biology and of medicine) to block the NPC1 lock. There was just one problem: Both protein targets lay deep within the lysosomes of host cells, seemingly shielded from antibody attack.
To overcome that hurdle, the researchers came up with an ingenious Trojan horse strategy that used the viruses themselves to carry the antibodies into the lysosomes. Just as the citizens of Troy unwittingly pulled a wooden horse filled with Greek soldiers into their walled city, the researchers tricked the viruses into carrying the two monoclonal antibodies with them as they traveled deep into host cells.
The strategy crucially required a third antibody as well—FVM09—which binds to glycoproteins on the surface of filoviruses. The researchers grafted the FVM09 monoclonal antibodies onto the anti-lock antibodies as well as the anti-key antibodies, creating two types of “bispecific” antibodies (i.e., synthetic antibodies with two “business ends” that allow them to bind to two different antigens). With their FVM09 business ends, both bispecific antibodies attach to the virus while it’s in the bloodstream, allowing the antibodies to hitch a ride on the virus as it infects a cell and gets carried into the lysosome. Once inside the lysosome, the lock and key business ends of the bispecific antibodies swing into action and bind to their key and lock targets.
Tests have shown that these bispecific antibodies are highly effective in preventing Ebola infection in human cells and in mice. In the future, the antibodies will need to be tested in nonhuman primates, the current gold standard for anti-Ebola therapeutics.
Whether the antibodies will be highly effective in humans is an open question, but even a modest boost to the immune system may be enough to contain an Ebola infection. “With acute diseases like hemorrhagic fevers, it’s simply a race to protect the body long enough so it can mount its own immune response,” says Dr. Lai, co-leader of the study. “Research on Ebola infection in nonhuman primates shows that, two weeks after infection, they can generate their own antibodies and clear the Ebola virus.”
Billions of people are at risk for sores caused by two closely related herpes viruses—herpes simplex 1 (HSV-1) and herpes simplex 2 (HSV-2). Both types spread via contact with the skin or mucous membranes of an infected person. After infection, both live in an inactive form in the ganglia (clusters of nerve-cell bodies) of sensory nerves. There they periodically reactivate and multiply, causing new sores that increase the likelihood of infecting other people.
Oral herpes appears as cold sores or fever blisters on or around the mouth and affects two-thirds of the world’s population. It is usually caused by HSV-1, which also causes more-serious health problems, including infectious encephalitis and corneal blindness.
Genital herpes is contracted mainly through sexual activity and is traditionally associated with HSV-2 (although in the United States and other developed countries, up to half of all new genital herpes infections are now from HSV-1). The classic genital herpes symptoms are small blisters that break open and cause painful sores. HSV-2 infections are especially likely to reactivate. They cause an average of four to five outbreaks each year, and the resulting sores greatly increase a person’s risk for HIV infection. With more than 400 million people infected with HSV-2 (some 48 million of them in the United States), the virus plays a major role in fueling the HIV/AIDS epidemic.
Until now, no drugs have been found to eradicate either type of HSV infection and no vaccine to protect people against them, but that may soon change. At Einstein, a radically different approach to vaccine development has led to a vaccine candidate that shows promise for preventing—and possibly treating—both viral scourges.
The immune system responds to viral infections by producing specific antibodies against the invading pathogen. The immune system also develops a “memory” of the virus, usually conferring lifelong immunity should that virus ever appear again. Vaccines against certain viruses work the same way. They contain viral proteins known as antigens that stimulate the immune system and prepare it to rapidly produce antibodies against the virus, even if actual exposure doesn’t occur until years later.
But an effective HSV vaccine has proved elusive. Scientists who developed the half dozen vaccines tried so far have all assumed that an effective HSV vaccine must contain a particular antigen known as glycoprotein D (gD-2). It’s found on the surface envelope of HSV-1 and HSV-2 and is used by the viruses to infect human cells. A gD-2 glycoprotein vaccine would presumably work by stimulating the immune system to produce neutralizing antibodies (which destroy targets without help from other parts of the immune system). In large-scale clinical trials, those vaccines did trigger high levels of neutralizing antibodies but failed to protect against HSV infection or disease, prompting Einstein researchers to adopt an entirely different strategy.
Rather than focus on gD-2 to stimulate antibodies, the Einstein team instead based its vaccine on HSV-2 viruses from which they deleted the gene that codes for the gD2 protein.
“We did this partly out of desperation and partly out of curiosity,” says team co-leader William Jacobs, Jr., Ph.D., a Howard Hughes Medical Institute investigator, the Leo and Julia Forchheimer Chair in Microbiology and Immunology and professor of microbiology & immunology and of genetics. “But we did have a hunch that gD-2 is such a dominant antigen that the immune system focuses on responding to it while ignoring other antigens. So removing this dominant protein might expose those previously masked antigens to the immune system and stimulate the body to produce different and more-effective antibodies.”
The altered virus strain was dubbed “delta-gD-2.” (“Delta” is shorthand for a gene deletion.) The vaccine containing it did not trigger the high levels of neutralizing antibodies that other experts had insisted were vital—but it worked spectacularly well in animal tests. The new vaccine had instead triggered copious amounts of antibodies associated with a different immune response called antibody-dependent cell-mediated cytotoxicity (ADCC). In ADCC, antibodies bind to antigens on the target’s surface; immune-system cells then recognize the target-bound antibodies and destroy the target.
When the vaccine was given to mice or guinea pigs, it completely protected them against subsequent infection with either HSV-1 or HSV-2, says team co-leader Betsy Herold, M.D., the Harold and Muriel Block Chair in Pediatrics at Einstein and chief of the division of pediatric infectious diseases at Children’s Hospital at Montefiore and Einstein. Moreover, she notes, the gene-deleted virus in the vaccine did not multiply in the animals, which meant that the vaccine was safe.
Vaccinated mice later exposed to HSV-1 or HSV-2 had no detectable virus in the vagina, the skin or neural tissue, where the viruses lurk in latent form and often emerge later to cause an outbreak. By contrast, unvaccinated mice challenged with HSV-1 or HSV-2 all showed evidence of the virus in those three sites, and all developed disease. This showed that ADCC antibodies were responsible for protecting against both types of herpes virus.
Nevertheless, reviewers were skeptical when the researchers submitted their findings to a leading journal. As noted above, the new vaccine didn’t trigger neutralizing antibodies. “There was a chorus of comments insisting that ‘everybody knows that an efficacious herpes vaccine requires neutralizing antibodies,’” Dr. Jacobs recalls.
Now the scientific community seems to be coming around to Dr. Jacobs’ and Dr. Herold’s unconventional approach to vaccinology. Their results were published in 2015 in eLife and in 2016 in the Journal of Clinical Investigation Insight. Also in 2016, the researchers were awarded a three-year, $3.1 million grant from the National Institute of Allergy and Infectious Diseases for further studies. “It has made people go back to the drawing board and recognize that inducing ADCC may be an important vaccine strategy for other viral pathogens as well, including HIV,’” says Dr. Herold.


The Einstein team recently showed that their vaccine protects mice and other animal models against an array of HSV-1 and HSV-2 samples provided by Amy Fox, M.D., M.S., professor of pathology and of pediatrics at Einstein and director of Montefiore’s clinical virology lab. Plans are under way to assess the vaccine in phase 1 clinical trials.
The team hopes its work could also lead to a therapeutic vaccine for people already infected with HSV. “If you could reduce the number of outbreaks from, say, six a year to one a year, that would make a lot of patients very happy,” says Dr. Herold. “And just maybe, we could prevent the virus from ever reactivating, or eliminate the reservoir of virus altogether.”
Dr. Jacobs, whose research career has focused mainly on tuberculosis, is now studying how to adapt this vaccine to fight TB—particularly latent TB infection, where the mycobacterium hides indefinitely within immune cells known as macrophages, only to emerge years later when the immune system is compromised (due to aging, for example, or to infection with HIV).
“The HSV vaccine selectively induces ADCC,” he says. “So if we can figure out what proteins are expressed on the surface of macrophages infected with TB, we should be able to develop an HSV vaccine that stimulates the corresponding antibodies, which would presumably induce an ADCC response against the infected macrophages.”
Of the hundreds of viruses that sicken humans, few sound more exotic than chikungunya (pronounced CHE-kun-gun-ya). This mosquito-borne virus causes fever and debilitating joint pain, which can persist for months or years. The disease was first observed in 1952 in present-day Tanzania. Locals named it “chikungunya,” which in the native patois means “that which bends up”—a reference to victims’ contorted posture due to the severe aches and pains caused by the infection.
Chikungunya has long infected millions of people in the tropics and subtropics each year. But in 2016, several dozen cases were reported in the United States—mostly among travelers returning from affected regions, although local transmission was found along the U.S. southern border. Public health officials aren’t panicking just yet. But thanks to climate change and global travel, chikungunya may not remain exotic to Americans much longer. Plus, there’s no vaccine and no treatment.
Margaret Kielian, Ph.D., professor of cell biology and the Samuel H. Golding Chair in Microbiology, is among a handful of researchers worldwide who study chikungunya—one of many disease-causing viruses that are enveloped viruses, meaning they’re surrounded by lipid membranes containing viral proteins. During infection, all enveloped viruses must fuse their membranes with some part of the host cells. Over the years, her lab has studied how chikungunya and other enveloped viruses enter and exit cells—vital information for developing vaccines and antiviral therapies.
In a study published in December 2016 in PLoS Pathogens, Dr. Kielian and her Einstein colleague Maria Guadalupe Martinez, Ph.D., associate in cell biology, described for the first time how alphaviruses (which include chikungunya) are transmitted from cell to cell during infection. Alphavirus infection dramatically alters a host cell’s cytoskeleton—the microtubules and other proteins that support cells and help maintain their shape. The infected cells send out long, thin “fingers,” or extensions, that touch uninfected neighboring cells, enabling the virus to travel from one cell to another.
“This mode of viral transmission—using these extensions to travel from an infected cell to a noninfected cell—may effectively shield some copies of the virus from host antibodies that could neutralize them,” says Dr. Kielian. “This may explain why symptoms can persist for so long and why conventional vaccines have thus far failed to efficiently protect against infection. By learning more details of this mechanism, we might be able to find new targets for antiviral therapies or develop effective vaccines for chikungunya.”
The standard way to make vaccines against viruses—find antigens that will prime the immune system to beat back infection—doesn’t work against dengue fever, a painful and debilitating mosquito-borne disease that affects tens of millions of people a year, primarily in tropical and subtropical areas.
The problem: there are four types of dengue, and becoming infected with one doesn’t confer protection against the others, explains Dr. Kielian, a dengue expert. Even worse, a second infection with a different type of dengue can cause a potentially deadly reaction called antibody dependent enhancement (ADE). In ADE, antibodies made against the first type of dengue bind to the second type of dengue, creating complexes that actually help the virus infect cells more efficiently. This leads to a supercharged infection that causes widespread inflammation that can lead to a form of septic shock—a life-threatening complication of infection.
A dengue vaccine, like dengue infection itself, could also lead to ADE unless it’s designed to produce antibodies that neutralize all four types of dengue. A recently licensed vaccine has proven somewhat effective against three of the four types. Drs. Kielian and Lai are working to create a better and truly universal dengue vaccine, using an approach called reverse vaccinology. Rather than starting with a piece of the virus that can stimulate production of neutralizing antibodies, researchers using reverse vaccinology work backward, starting with the antibodies.
“If you have an antibody that you know can neutralize a virus or other pathogen, in theory you can engineer an antigen protein that binds to that antibody,” says Dr. Lai. “If that antigen were injected into a person who had never been infected, it would stimulate the production of protective antibodies. Then you’d have a vaccine.”
Researchers had previously identified two broadly neutralizing antibodies
that bind to a particular section of the viral envelope present in all four types of dengue—an important step in reverse-engineering a dengue vaccine. Drs. Lai and Kielian used sophisticated protein-engineering techniques to look more closely at this viral section where antibodies interact with all four types.
The Einstein scientists found differences in the way the antibodies interacted with each type of dengue; notably, they found that both antibodies interacted with some viral regions shared by all four types of dengue. Their findings, published in Virology in 2015, provide important new clues for choosing precisely the right viral components for a vaccine that will work against all four types of dengue virus.
Disease-causing viruses likely will always be with us. But thanks to the work of Einstein-Montefiore scientists, new strategies should be available for treating or preventing some of these diseases—the sooner the better.