
Error: No layouts found
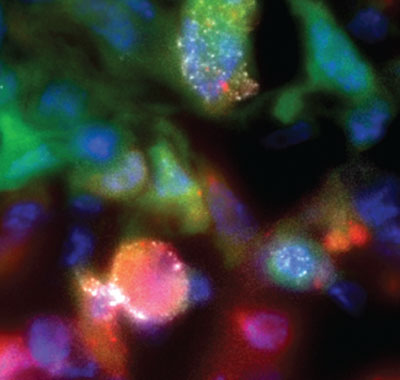
Most cancer deaths result from breakaway tumor cells that spread, or metastasize, to distant organs. Prime suspects are hypoxic (oxygen-deprived) cells that overexpress genes involved in cancer-cell invasion and metastasis. A study by Einstein researchers, published last year in IntraVital, offered unprecedented insights into their behavior. After designing a red fluorescent protein that turns on under low-oxygen conditions, the researchers used it to label breast cancer cells, and—using multiphoton microscopy—compared hypoxic and “normoxic” tumor cells (labeled with green fluorescent protein) at single-cell resolution in live breast xenograft tumors (human tumor cells growing in mice). To metastasize, tumor cells must first degrade collagen and other substances outside the cell to reach nearby blood vessels. This image shows the increased activity of hypoxic cancer cells in tumors. The bright white spot on the red (hypoxic) cell shows it was actively degrading collagen. The work was done in Einstein’s Gruss Lipper Biophotonics Center. The paper’s lead author was Yarong Wang, M.S., an associate in the department of anatomy and structural biology; senior author John S. Condeelis, Ph.D., is a professor and co-chair of the department.