Error: No layouts found
While growing up in Spain, Ana Maria Cuervo, M.D., Ph.D., often heard her mother say, “In a clean house, everything works better.” This sage advice would become the guiding principle of her scientific career.
Dr. Cuervo is a physician-scientist who specializes in autophagy—from the Greek words “auto” and “phagein,” meaning “self-devouring.” Essentially, autophagy is a fancy term for cellular waste management. Almost every type of cell in the human body uses a variety of strategies to degrade and recycle old, mutated or otherwise damaged proteins and other molecules so that cells’ interiors remain spic-and-span.
Autophagy was discovered in the 1960s, but the biomedical community paid little attention at first. After all, who cares about the trash as long as it’s discarded regularly? But over time, researchers realized that good cellular housekeeping is not just for neat freaks but also maintains health by preventing defective proteins from accumulating inside cells. What’s more, since autophagy slows down with age—one of Dr. Cuervo’s major discoveries—therapies to enhance autophagy may help prevent or reverse a range of age-related diseases and perhaps slow the aging process itself.
Autophagy is finally basking in the limelight. In October, the Japanese scientist Yoshinori Ohsumi was awarded the 2016 Nobel Prize in Physiology or Medicine for his pioneering studies of the genes and mechanisms that underlie this vital process.
“The honor recognizes our field of autophagy and how basic research—Dr. Ohsumi studies yeast genes—can have a transforming impact on biomedical science,” says Dr. Cuervo, a professor of developmental and molecular biology, of anatomy and structural biology and of medicine; co-director of the Institute for Aging Research; and the Robert and Renée Belfer Chair for the Study of Neurodegenerative Diseases at Einstein.
“The discovery of the autophagy genes in yeast has allowed researchers including me to establish connections between autophagy failure and many human disorders, including cancer, diabetes and Alzheimer’s disease,” she says. “Academic and industry labs are now developing drugs that target those genes, with the goal of curing those diseases by modulating autophagy.”

Ana Maria Cuervo, M.D., Ph.D.
Early honors
Dr. Cuervo won recognition early in her career for dispelling the notion that lysosomes—the cells’ enzyme-filled recycling centers—degrade proteins at random. She and her mentor (the late J. Fred Dice of Tufts University School of Medicine) discovered that this recycling effort is actually quite selective and identified the molecular machinery that drives the process, which they dubbed chaperone-mediated autophagy (CMA). It involves specialized proteins that guide, or “chaperone,” old and damaged proteins to the lysosomes for digestion.
“CMA makes sure your proteins behave—kind of like the old-fashioned chaperone who escorted you and your boyfriend to the movies,” says Dr. Cuervo. “And if the proteins don’t behave, CMA eliminates them from the cell.” Those findings were published in the journal Science in 1996, a coup for such a young scientist.
Since joining the Einstein faculty in 2001, Dr. Cuervo has made several other major discoveries in the field:

The making of a scientist
Most parents are thrilled to hear that their child is applying to medical school. Ana Maria Cuervo’s were dumbfounded. “You remember that you faint when you see blood?” her mother reportedly asked.
“That was true,” admits Dr. Cuervo, who was born in Barcelona and raised in Valencia. She applied anyway, reasoning that medical school was the best path to a career in research, her ultimate destination. She had little company. “Just three of us in our class of 300 at the University of Valencia were interested in research,” she recalls. “They were my best friends, and one of them became my husband [Fernando Macian-Juan, M.D., Ph.D., now a professor of pathology at Einstein].”
Dr. Cuervo grew accustomed to the sight of blood, but her days at the bedside only reinforced her plans to do research. “It was shocking,” she says. “I am very empathic, and the first thing they taught us in medical school was that you cannot be, or you will be suffering with all your patients.” She also came to realize that she had less interest in learning how things are done in medicine and more in discovering how they could be done better.
After graduating from medical school in 1990, she set her sights on a doctorate in biochemistry and molecular biology and looked for a lab studying the biology of aging. “During my rotation in geriatrics, I realized that almost nothing could be done for older people other than helping manage their symptoms,” she explains. “It was the most depressing thing I had ever seen.”
Dr. Cuervo was initially directed to a lab at the Instituto de Investigaciones Citológicas, also in Valencia, which focused on the role of mitochondria (the cell’s powerhouses) in aging. As fate would have it, the principal investigator was absent the day of her visit, so she was sent to the office of Erwin Knecht, a lysosome specialist.
Dr. Knecht invited her to join his team, but it wasn’t the warmest of welcomes. When his lab switched its emphasis to proteasomes—supposedly the next big thing in protein degradation—she was told to stick with lysosomes. “Dr. Knecht had never before had an M.D. in his lab and wasn’t sure if I was going to continue a career in science,” she says. “That was hard for me. All the cool things were happening in proteasomes.”
By 1993, Dr. Cuervo published her first paper on lysosomes, the one demonstrating the selective nature of autophagy. The budding scientist’s next break came shortly thereafter, thanks to Spain’s custom of closing down most workplaces, labs included, for the long summer holiday.
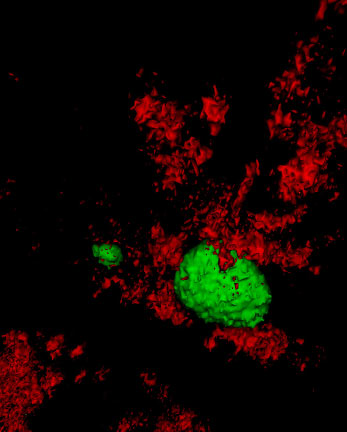
The autophagy chaperone (red) removes proteins covering fat deposits (green) so the fat can be metabolized for energy.
Instead of taking time off, Dr. Cuervo ventured to Tufts to work with Dr. Dice, an acquaintance of Dr. Knecht’s and a fellow autophagist. One summer morphed into two and then into three. Then, Ph.D. degree in hand, she crossed the Atlantic one more time to join the Dice lab as a postdoc in 1994, despite warnings from colleagues that autophagy was a career dead end.
It almost was—for another reason. Soon after Dr. Cuervo arrived at Tufts, Dr. Dice began losing his eyesight from complications of diabetes and offered to find his protégé a spot in another lab. She declined, even as the lab shrank and its funding dwindled. For six years, the two collaborated, she from the lab, he from home, leading to their discovery of CMA and their groundbreaking finding in 2000 that CMA naturally declines with age in mammals, opening a whole new perspective on the biology of aging.
Thanks in part to Dr. Cuervo’s own contributions, lysosomes were becoming “cool.”
A fountain of youth?
After relocating to Einstein, Dr. Cuervo discovered why CMA declines with age: As animals get older, a receptor on the lysosomal membrane known as LAMP-2A—which helps pull chaperone-delivered proteins into the lysosome for digestion—progressively disappears.
To see whether this CMA decline could be halted, Dr. Cuervo designed a transgenic mouse model in which the age-related decrease in LAMP-2A was prevented. The results, published in Nature Medicine in 2008, showed that the transgenic mice maintained CMA activity until advanced ages. And compared with “normal” aging animals, the aged transgenic mice had less intracellular accumulation of damaged proteins and improved liver function—the first evidence in mammals that preventing the age-related decline in autophagy slows cellular aging and preserves organ function.
Dr. Cuervo’s work has since expanded into neurodegenerative disorders, and she and her collaborators have found evidence that CMA is impaired in Parkinson’s and Alzheimer’s diseases.
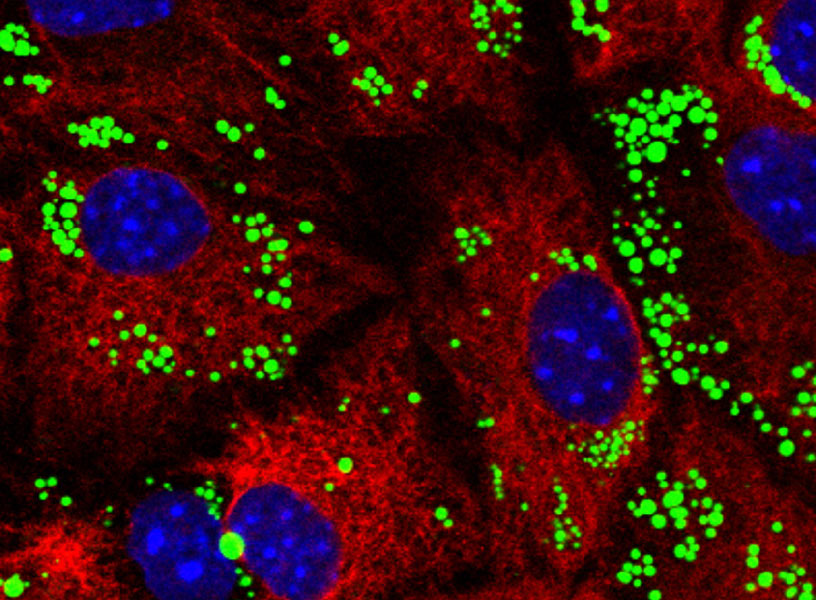
In mouse brain cells, fat deposits (green) have accumulated because a type of autophagy called lipophagy has failed to metabolize them.
“Healthy older individuals can lose, say, 50 percent of their ability to recycle materials and they’ll still do reasonably well,” says Dr. Cuervo, whose words tumble out by the dozens as she enthusiastically discusses her favorite topic. “But problems can arise for people who produce mutant proteins—as in some forms of Alzheimer’s disease—but whose efficiency at getting rid of those proteins decreases significantly. This leads mutant proteins to accumulate even as their rate of production stays the same, ultimately contributing to neurological problems.”
Studies suggest that other intracellular stressors that affect older people—the elevated blood glucose of type 2 diabetes and abnormally high lipid levels of atherosclerosis, for example—similarly overwhelm the cells’ ability to clear them.
While autophagic aging may be inevitable, the good news is that it may be treatable. A study led by Dr. Cuervo’s husband, Dr. Macian-Juan, found that centenarians have naturally robust autophagy, which presumably contributes to their longevity. “If we can determine the genes that are responsible for their youthful autophagy, we might be able to mimic this effect with drugs or other therapies,” says Dr. Cuervo.
Dr. Cuervo’s research team is already testing autophagy-boosting drugs, which have shown promise in animal models. In the meantime, people of any age can take measures to keep their autophagy humming along. “Basically, follow your grandmother’s advice: eat a balanced diet, get enough sleep and exercise,” she says. “Each of these activates autophagy.”
Autophagy and cancer
Venturing even farther afield, Dr. Cuervo’s team is also investigating autophagy’s role in cancer, which affects primarily older people.
“As you age, the decline in autophagy’s efficiency makes your cells—particularly your DNA—more vulnerable to damage,” she explains. “And this increases the possibility of a normal cell transforming into a bad cell—a cancer cell.” Recently, Dr. Cuervo found that autophagy plays yet another role in cancer: Cells ramp up their CMA when they become malignant. Of 17 types of tumor cells that her lab analyzed, CMA was enhanced in almost every one. When the researchers blocked CMA, the tumors shrank, Dr. Cuervo reported in a 2011 paper in Science Translational Medicine.
“Since cancer cells have such a high rate of growth, they need a huge amount of nutrients, which they get through efficient recycling of their components,” she notes. “This would also explain why cancer cells can be so resistant to chemotherapy. Chemo aims to cause damage to the cancer cells. But because their CMA is so good, cancer cells can handle that damage very well. It’s as if they’re saying, ‘Bring it on!’”
In one of her many studies funded by the National Institutes of Health, Dr. Cuervo is attempting to find out how cancer cells activate CMA. “The CMA activation we see in cancer cells is what we’d like to achieve in normal cells,”she says.
“I’m not claiming that ‘fixing’ autophagy will be a cure-all, but it may do a lot,” says Dr. Cuervo. “Autophagy is a fundamental mechanism in the cell. And if the cell is cleaning up after itself, other things in the body will start to function more efficiently.”
Which is essentially what her mother said all those years ago.