Scientists have been poking and prodding the brain for centuries in hopes of learning how this gelatinous mass of billions of interconnected neurons influences thoughts, emotions, movement, mental and behavioral problems, and just about everything else that makes us human.
One of the great advances in neuroscience came in the 1930s, when a surgeon named Wilder Penfield used electrodes to explore the brains of epilepsy patients. Stimulating different parts of the brain with electricity revealed which regions control what movements, allowing him to identify areas to avoid during surgery. Penfield’s findings led to the first functional map of the motor areas of the brain.
Until recently, electrical stimulation remained the go-to method for studying the brains of experimental animals, revealing the actions controlled by different parts of the brain and their various specialized neurons. But the technique had serious drawbacks. Inserting electrodes into the brain can damage the very cells under study. And electrical stimulation was imprecise and nonselective, so it was impossible to know exactly which neurons were being activated.
In 1979 Francis Crick (who by then had turned his attention from DNA to neuroscience) made a suggestion: Researchers hoping to decode the brain would need to manipulate one type of neuron without altering any of the others. Light, he surmised, could provide a precise, nondestructive solution. Crick was on to something, but it would take decades for science to catch up.
The first component of this futuristic tool was already at hand. In the early ’70s, researchers discovered that certain microorganisms contain opsins—light-sensitive proteins that regulate the flow of an electric charge across cell membranes. Thanks to virus-mediated gene delivery, developed in the 1980s, the genes for opsin proteins could be inserted into neurons; when those genes become activated, the cells start synthesizing light-sensitive opsin proteins.
The final key component: the availability of long, slender optical fibers (similar to the kind that bring the Internet into homes) that could shine pulses of light on opsin-containing neurons almost anywhere in the brain. With the literal flick of a switch, this marriage of optics and genes—“optogenetics”—could now turn neurons and other cells in a living organism on or off (depending on the opsin).
In 2010, Nature Methods chose optogenetics as its Method of the Year. By now the technique has found its way into thousands of laboratories around the world. Here’s how four labs at Einstein are using the transformative effects of light to illuminate science.
“The idea that the cerebellum did much beyond controlling movement was met with considerable skepticism—and no one had any real clues as to how the cerebellum might affect dopamine release,” says Dr. Khodakhah, professor and chair of the Dominick P. Purpura Department of Neuroscience and the Florence and Irving Rubinstein Chair in Neuroscience.
For Dr. Khodakhah, all signs pointed to an as-yet-undiscovered link between the cerebellum and the ventral tegmental area (VTA), a nearby structure known to play a role in addiction. VTA neurons synthesize and release dopamine into the mesolimbic pathway, which mediates pleasure and reward. “However,” he says, “conventional tools for looking at brain anatomy could never tell us whether cerebellar neurons directly connected with the VTA, or if they simply passed by en route to other destinations.”
Dr. Khodakhah and his colleagues turned to optogenetics to provide the answer. Their research involved inserting opsin genes into mouse cerebellar neurons, which then processed the genes into light-sensitive opsin proteins. Exposing those neurons to light would selectively activate or inactivate the treated neurons, depending on the particular opsin used.
In an initial experiment, Dr. Khodakhah’s team inserted opsin genes into certain cerebellar neurons: those whose long fibers, known as axons, connected with the VTA. When these neurons were exposed to light, the VTA responded with measurable electrical activity. Since only opsin-containing cerebellar neurons could have been activated by the light, this experiment proved for the first time that cerebellar neurons form working synapses (connections) with VTA neurons.
To see whether those connections influence behavior, Dr. Khodakhah conducted a so-called open-field chamber test, in which mice were free to explore any corner of a square enclosure. Each time a mouse reached a particular corner (randomly chosen for each mouse), cerebellar neurons linked to the VTA were optogenetically stimulated.
If the mice found this stimulation pleasurable, they’d be expected to preferentially return to this corner (to get another rewarding flash of light) instead of to the other corners—and they did, much more so than occurred with control animals. (See images below.)
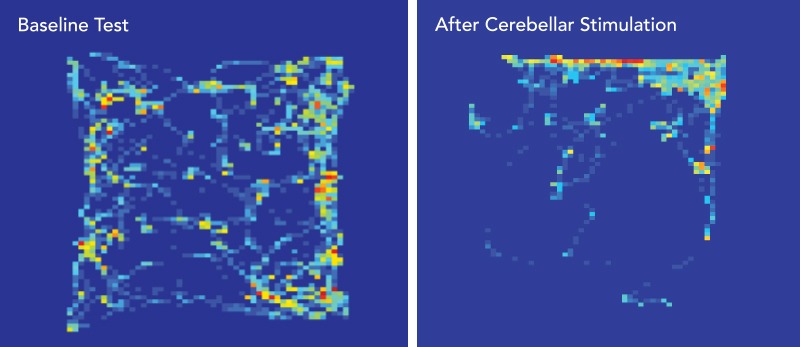 STIMULATING THE REWARD CENTER. Heat maps show how much time a mouse spends exploring the four corners of a square enclosure (warmer colors equal greater number of visits). Map at left shows time spent in the four corners before researchers optogenetically stimulated cerebellar axons in the mouse’s VTA whenever it entered the upper-right quadrant. At right, the mouse preferentially returns to the upper-right corner, presumably hoping for more pleasurable stimulation.
STIMULATING THE REWARD CENTER. Heat maps show how much time a mouse spends exploring the four corners of a square enclosure (warmer colors equal greater number of visits). Map at left shows time spent in the four corners before researchers optogenetically stimulated cerebellar axons in the mouse’s VTA whenever it entered the upper-right quadrant. At right, the mouse preferentially returns to the upper-right corner, presumably hoping for more pleasurable stimulation.
Could stimulating cerebellar projections to the VTA trigger “addiction” in mice? To find out, Dr. Khodakhah and colleagues put mice in a chamber that was half dark and half brightly lit. Since mice prefer dark areas—the better to avoid becoming a predator’s next meal—they spent more time exploring the dark part of the chamber.
The researchers then repeated the experiment—except this time, every other day for six days, mice were confined to the bright side for 30 minutes while cerebellar axons with connections to the VTA were optogenetically stimulated. After that initial conditioning period, the mice were allowed to freely explore the entire chamber.
Even though mice normally shun bright areas, now they ran toward the light, because that’s where they remembered getting a reward.
– Dr. Kamran Khodakhah
“Even though mice normally shun bright areas, now they ran toward the light, because that’s where they remembered getting a reward,” Dr. Khodakhah says. “This suggests that the cerebellum plays a role in addictive behaviors.” He notes that the results were “very similar” to findings in other studies in which mice confined to the bright part of chambers received addictive drugs, such as cocaine, instead of cerebellar stimulation.
Cerebellum abnormalities have been implicated in autism spectrum disorder (ASD), although how the cerebellum contributes to ASD isn’t clear. Because the VTA is required for social behavior, Dr. Khodakhah and colleagues tested whether the cerebellum-VTA pathway might be involved. They placed mice in a three-chambered box in which they were free to travel to either an inanimate object, another mouse, or an empty chamber. The activity of cerebellar axons within their VTA was monitored.
The mice being studied typically spent most of their time socializing with other mice—and when they did, cerebellar axons in their VTA were most active, consistent with the idea that the cerebellum relays information relevant to social behavior to the VTA. Intriguingly, when researchers optogenetically silenced cerebellar axons projecting into the VTA, the mice no longer preferred interacting with other mice.
This finding suggests that social behavior requires a functioning cerebellum-VTA pathway and that interference with this pathway may be a glitch through which cerebellar dysfunction contributes to ASD. “It would have been extremely difficult to make these discoveries without this technique,” Dr. Khodakhah notes.
Dr. Autry-Dixon recently identified a group of neurons in the mouse hypothalamus that is active during pup-directed aggression but not during normal parental behavior. In males, the use of optogenetics to silence those cells blocked pup-directed aggression, while optogenetically activating the neurons in females led to reduced maternal behavior and, in some cases, pup-directed attack—which normally is extremely rare among mouse mothers. She speculates that these neurons sit at the middle of a “social-stress circuit” in males and females and that, under certain conditions, the neurons misfire and lead to aberrant parenting.
Now Dr. Autry-Dixon is defining the anatomy and function of this possible social-stress circuit in mice to determine how these neurons control pup-directed aggression. Again, optogenetics will play a key role in her research. Her findings may shed light on neurobiological mechanisms underlying filicide and could lead to new treatments for parenting-related disorders, such as postpartum depression and postpartum psychosis, that can affect both mothers and fathers.
“It’s interesting how much our neural circuits change in response to parenting,” she says. “In female mice, for example, a lot of oxytocin activity occurs in the brain’s auditory cortex, allowing mothers to respond quickly to pups’ vocalizations. I’m the same way with my 4-month-old and wake up immediately when I hear my baby cry. My husband asks, ‘Was the baby up last night?’ And I say, ‘Yeah, six times!’ Mothers’ sense of smell also changes throughout pregnancy and early motherhood, and none of us would be here if humans didn’t have some kind of parenting instinct.”
Dr. Autry-Dixon likes to frame her work in a larger context. “What has struck me is that the cultural narrative around motherhood differs so starkly from the actual experience,” she says. “There’s so much pressure on mothers but often no maternity leave to help them bond with their infants, and little support from our healthcare system. So the inability of some mothers to form a strong maternal-infant bond is driven mainly by societal flaws rather than being a mother’s ‘fault.’ That recognition could lessen the stigma preventing parents from getting the help they need.”
Brown fat is found mainly in the neck and shoulder regions of hibernating animals and many small mammals—including newborn humans, who lack the ability to shiver in response to cold. The small clusters of brown fat that linger into adulthood were assumed to be physiologically unimportant.
But in 2009, three reports in The New England Journal of Medicine found not only that healthy adults possess significant amounts of brown fat but that the fat is also metabolically active. In one of the reports, exposing human volunteers to cold revved up their brown-fat activity fifteenfold, as measured by increased glucose uptake from the bloodstream (a reflection of brown-fat cells’ high metabolic rate).
“Brown fat’s ability to clear glucose means that activating it could help in treating or even preventing diabetes,” says neuroscientist Young-Hwan Jo, Ph.D., associate professor of medicine and of molecular pharmacology. “Activated brown fat also expends energy by burning off large numbers of calories—and the resulting weight loss can especially help people with diabetes, as well as anyone who is overweight.”
Dr. Jo’s research has shown that optogenetics could be a promising alternative to freezing temperatures for activating brown fat. In a study published in Molecular Metabolism in 2015, he and colleagues inserted opsins into mouse neurons in a region of the hypothalamus and then used optical fibers to stimulate those neurons. The study conclusively demonstrated that neurons in the hypothalamus regulate brown-fat metabolism.
Optogenetics could be a promising alternative to freezing temperatures for activating brown fat.
“This procedure is too invasive for humans, of course,” Dr. Jo says. But three years later, in a 2018 mouse study published in Scientific Reports, Dr. Jo used a noninvasive optogenetic technology to stimulate peripheral autonomic neurons. These nerves, in the back of the necks of mice, connect with brown fat directly below.
“We knew that blue light readily penetrates human and rodent skin,” Dr. Jo says. “So we expressed opsins in the neurons and then shone blue light directly through the skin.” The underlying brown fat was successfully activated, as shown by nonshivering thermogenesis and lowered blood-glucose levels in the mice, reflecting metabolically active brown-fat cells taking up glucose from the bloodstream.
Dr. Jo and Einstein have applied for a patent on his noninvasive technology, which uses a computer-controlled pulse generator to emit bursts of light-pulses. Applications for Dr. Jo’s technology could extend beyond brown fat, because it can stimulate (or turn off) any nerves close to the skin surface.
“For example,” Dr. Jo says, “we know that autonomic nerves in the liver play a crucial role in regulating the body’s blood-glucose concentrations. We hope that optogenetically stimulating those nerves can normalize the excessive blood-glucose levels in people with diabetes.”
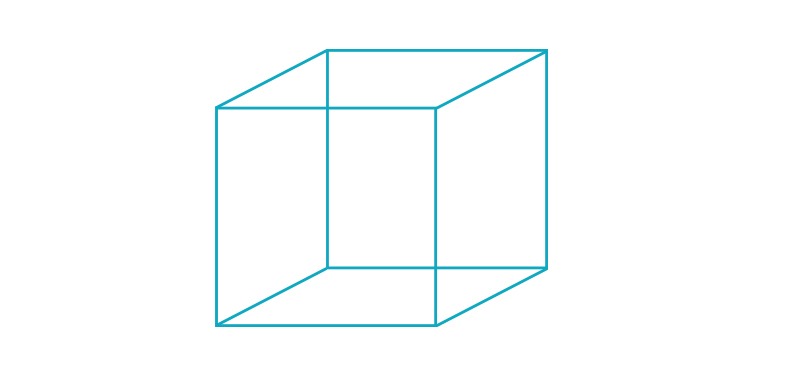 Above, the Necker cube optical illusion, first published in 1832 by Swiss crystallographer Louis Albert Necker. The cube’s lower-left square or its upper-right square may appear closest to you—or they may switch back and forth as you stare. Feedback signaling in the visual cortex may influence our perception of reality by causing the brain to make the sort of inferences seen in this and other optical illusions.
Above, the Necker cube optical illusion, first published in 1832 by Swiss crystallographer Louis Albert Necker. The cube’s lower-left square or its upper-right square may appear closest to you—or they may switch back and forth as you stare. Feedback signaling in the visual cortex may influence our perception of reality by causing the brain to make the sort of inferences seen in this and other optical illusions.
Until recently, the only way researchers could assess the influence of feedback neurons on vision was to inactivate them (using drugs or cooling probes, for example) and then monitor the effects. But the downstream consequences were subtle and difficult to interpret.
For one thing, it was hard to know exactly which neurons had been inactivated. Also, the timing of the inactivation couldn’t be controlled—a considerable downside in a circuit that works on a millisecond timescale. Finally, by the time the neurons were inactivated and the effects measured, the brain could already have compensated for the loss.
“Basically, we needed a way to control neurons that was instantaneous, reversible, and more precise,” Dr. Kohn says. “That was the promise of optogenetics.” Adapting that tool to vision would not be easy: Most optogenetics research had involved mice, which unfortunately are not good animal models for studying human vision. Nonhuman primates are ideal animal models for vision study, but little optogenetics research had been done on them.
“It took some time for us to learn how to get opsin genes into the right cells,” Dr. Kohn says. Once he did, he was able to selectively activate upper visual-cortex neurons while recording the effect on neurons in the lower cortex, a hundred neurons at a time.“If we use a musical analogy, what we could do before was hit the piano with a sledgehammer,” he adds. “What we can do now is use our fingers to play a few notes. But it would be really nice to be able to play a melody that mimics natural activity patterns in the brain and then observe the downstream effects.”
It’s too early for Dr. Kohn to draw conclusions from his preliminary experiments. “We’ve seen very robust effects caused by manipulating feedback neurons, which itself is a big step forward,” he says. “The firing rate of the affected neurons changes, and so does their activity. When feedback signals are active, the lower cortical neurons appear to change from fluctuating together—that is, acting redundantly—to functioning independently. If these independently functioning neurons are each carrying different bits of information, then they might provide us with a richer representation of the world. But we need to do more work to see if this is what is actually happening.”
Dr. Kohn’s initial data are in line with current theories that feedback signals aid tasks such as figure-ground segregation (helping you separate the tree from the field) and predictive coding (helping you predict future locations of a moving object). His studies could have important implications for human health. “Disruptions in signaling between areas of the visual cortex have been implicated in schizophrenia, autism, and several other disorders,” Dr. Kohn says. “Gaining a better understanding of feedback circuitry could yield insight into the underlying causes of those disorders.”