Who doesn’t have fond memories of the childhood puzzle Connect the Dots? With the stroke of a pencil, you could create order from disorder by transforming a seemingly random series of numbered points into a line drawing of a fish, a rabbit, or a tree. Einstein scientist Scott Emmons, Ph.D., has never outgrown his fascination with that sort of magic.
For almost two decades, Dr. Emmons, professor of genetics and in the Dominick P. Purpura Department of Neuroscience and the Siegfried Ullmann Chair in Molecular Genetics, has been connecting many thousands of dots—each representing a neural structure—visible on thousands of serial electron micrographs of the tiny roundworm Caenorhabditis elegans.
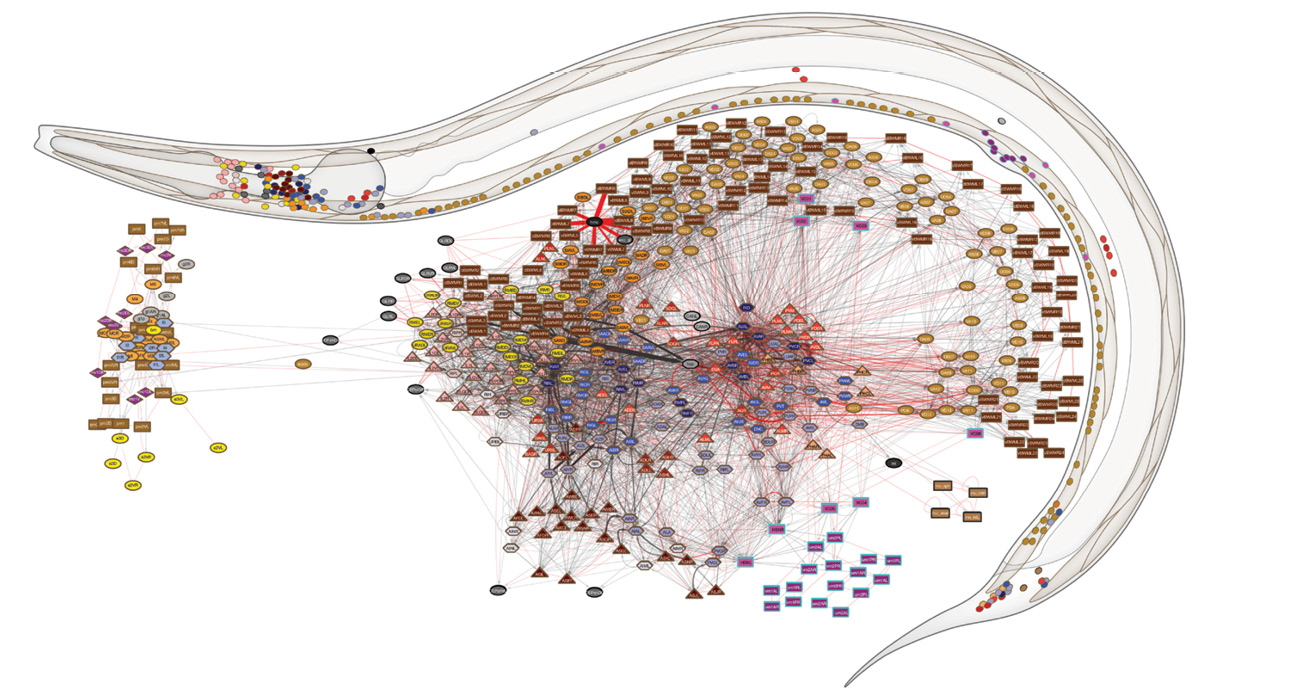
Dr. Emmons’ painstaking pointillist portraiture has culminated in the first complete wiring diagram of the nervous system of any animal—in this case, an animal used by scientists worldwide as a model organism for studying the basics of biology and disease.
Dr. Emmons’ magnum opus—featured on the July 4, 2019, cover of the prestigious journal Nature—marks a major milestone in the field of “connectomics,” the effort to map the myriad neural connections in a brain, brain region, or nervous system, with the goal of finding the specific nerve connections responsible for particular behaviors.
“Structure is always central in biology,” Dr. Emmons says. “The structure of DNA revealed how genes work, and the structure of proteins revealed how enzymes function. Now the structure of the nervous system is revealing how animals behave and how neural connections go wrong to cause disease.”
Although the word connectomics began to be used only some 15 years ago, the field dates back at least to the Renaissance. Fascinated with anatomy, Leonardo da Vinci dissected a variety of organisms, from frogs to oxen to humans, seeking to understand how the nervous system’s interconnected structures give rise to the senses and perhaps even the soul. With his crude tools and razor-sharp mind, he developed an original model of sensory physiology. (The soul, alas, eluded his grasp.)
Connectomics began in earnest in the 1960s with Sydney Brenner, a South African–born molecular biologist based at the Medical Research Council in Cambridge, England. Early in his career, Dr. Brenner made important discoveries regarding how genes replicate and convey information. In the late 1960s, he turned his attention to the genetic and neural bases of behavior.
Dr. Brenner’s first order of business was describing the structure of a nervous system: a network of connected neurons. He realized that figuring out how those neurons are connected would be essential (although not sufficient) for understanding how that nervous system functions. Or to paraphrase one neuroscientist: You won’t fully understand the brain with a wiring diagram, but you likely won’t understand the brain without one.
Dr. Brenner needed a suitable experimental organism, one with accessible genetics but relatively advanced behaviors. The fruit fly Drosophila met both criteria, but its 100,000-neuron nervous system was too complex. He ultimately chose C. elegans, a benign, nearly translucent, millimeter-long roundworm. It can be found the world over, dining on microbes that live on rotting vegetation, especially fruit. (“It really should be called the fruit worm,” Dr. Emmons says.)
Although C. elegans lacks circulatory and respiratory systems, its nervous system—which includes a rudimentary brain—is capable of several functions: basic learning, memory, experiencing fear, sensing and squirming away from predators, navigating toward food, and mating and reproducing using its sperm and eggs. All those activities occur in one tiny animal containing only about a thousand cells, one-third of them dedicated to the nervous system.
Although the worm was small, the task ahead loomed large. Details of its nervous system were beyond the resolving power of the light microscope, so Dr. Brenner and his colleagues would need to use electron microscopy (EM). EM works by passing electrons through razor-thin slices of tissue to produce magnified images. Some 20,000 cross-sectional slices—each one-thousandth the width of a human hair—would be required to cover the roundworm’s nervous system from “head” to “toe.”
 C. Elegans by the Numbers
C. Elegans by the Numbers
 1900
year first described as a species
1900
year first described as a species
 1–2 worms
able to fit on the head of a pin
3 Days
development from a fertilized egg to a fertile adult
1–2 worms
able to fit on the head of a pin
3 Days
development from a fertilized egg to a fertile adult
 C. Elegans by the Numbers
C. Elegans by the Numbers
 38%
C. elegans genes with human equivalents
38%
C. elegans genes with human equivalents
 NEURONS
383 in an adult male
NEURONS
383 in an adult male  synapses
~8,000 chemical & electrical connections
synapses
~8,000 chemical & electrical connections
 C. Elegans by the Numbers
Roundworm researchers WORLDWIDE
C. Elegans by the Numbers
Roundworm researchers WORLDWIDE

 15K-20K Electron Micrograph Slices
needed to construct the complete connectome
15K-20K Electron Micrograph Slices
needed to construct the complete connectome
 Nobel Prizes
awarded to C. elegans
Nobel Prizes
awarded to C. elegansThousands of laboratories around the world would adopt the roundworm as an essential animal model.
Further complicating things, each slice contained numerous anatomical details—slivers of chemical and electrical synapses and neuromuscular junctions. (A synapse is the junction where a neuron passes an electrical or chemical signal to another neuron, or where a neuron connects with another type of cell. For example, a neuromuscular junction is a synapse at which a neuron transmits a signal to a muscle fiber, triggering muscle contraction.)
Making a wiring diagram required scrutinizing every slice, identifying each neural structure, and connecting that structure to the corresponding structure in the previous slice—all by hand.
Led by lab member John White, a team of half a dozen scientists and artists worked for 15 years to connect most of the dots. All told, Dr. Brenner and his colleagues produced 132 maps, encompassing about 5,000 chemical synapses, 600 electrical synapses, and 2,000 neuromuscular junctions. In 1984, they delivered their Tolstoyan, 450-page manuscript—subtitled “The Mind of a Worm”—to Philosophical Transactions of the Royal Society. It was published two years later as a special one-volume issue of the journal.
The scientific community initially greeted the paper with a collective yawn, perhaps because so few researchers knew what to make of it. One reviewer confined his comments to the introductory discussion, writing “after all, few will read the detail.” But C. elegans research eventually flourished, largely because of all those connectome maps. Thousands of laboratories around the world would adopt the roundworm as an essential animal model. “‘The Mind of a Worm’ became their bible,” Dr. Emmons wrote.
The study of Dr. Brenner’s connectome has yielded numerous benefits, including the discoveries of:
Since its publication 36 years ago, Dr. Brenner’s paper has been cited more than 4,000 times—a level of recognition achieved by just a handful of studies. In 2002, Dr. Brenner was honored with a share of the Nobel Prize in Physiology or Medicine for findings related to his work on C. elegans. He died in 2019 at age 92.
Voluminous though it was, Dr. Brenner’s roundworm map was incomplete. It skipped large portions of the worm’s body and pertained only to the hermaphrodite (which can self-fertilize and is considered the equivalent of the female of the species), not to the male.
In 1999, Dr. Emmons and his colleagues took on the challenge of finishing Dr. Brenner’s map. Looking back, it seems as though Dr. Emmons’ career had been designed to lead him to this very task.
A native of Sudbury, Massachusetts, Dr. Emmons discovered his love for science and mathematics through his father, a professor of applied engineering at Harvard University. Dr. Emmons earned a B.S. in biology at Harvard, followed by a Ph.D. in biochemistry at Stanford, where he grew interested in gene function and regulation and developmental biology.
Shortly after earning his doctorate in 1974, he heard a lecture on C. elegans and quickly realized that this tiny organism could help him in his research.
Geneticists gain insights into how normal genes work by inducing mutations and observing their effects. So they prize animal models that have readily manipulable DNA, short life cycles, and quick reproduction—all of which the roundworm offers.
Dr. Emmons joined the Einstein faculty in 1979. Several years later he took a sabbatical to do a postdoctoral fellowship at the Medical Research Council, the mecca of C. elegans research, studying under Jonathan Hodgkin, Ph.D., one of Dr. Brenner’s former students.
Upon returning to Einstein in 1986, Dr. Emmons studied how genes encode roundworm morphology (form and structure), eventually focusing on male mating behavior and the neurons responsible. “It struck me that the male connectome was a big missing piece of C. elegans biology. Our field is characterized by completeness. We seek to know all the cells in the organism, all the genes in the genome, and all the connections in the nervous system,” Dr. Emmons says.
With perfect timing, the National Institutes of Health (NIH) issued a call for “big field” projects with the potential to affect a wide array of disciplines. To Dr. Emmons, his quest for the male roundworm connectome fit the bill. The NIH agreed, awarding him a pilot project grant to develop the necessary software. The project would ultimately be funded for 10 years by the G. Harold and Leila Y. Mathers Charitable Foundation.
“We thought it could be done with the help of a personal computer, but we really didn’t know,” Dr. Emmons recalls. “The male is much more complex than the female. Also, it was possible that some of the neurons ran in the plane of the slices rather than across them, which would mean we wouldn’t be able to see all the connections using serial electron micrographs.”
Dr. Brenner by this time had different research priorities and was pleased that others were continuing work on the C. elegans connectome, according to Dr. Emmons.
To complete the male connectome, Dr. Emmons would need an electron microscopist with expertise in roundworms. Such specialists were rare at the time, so he was incredibly lucky to find one at Einstein: David Hall, Ph.D., professor in the Dominick P. Purpura Department of Neuroscience.
Dr. Hall began working with C. elegans while earning his doctorate at the California Institute of Technology. He came to Einstein in the mid-1970s to study the comparative anatomy of gap junctions in animals, including fish and snails, but eventually switched back to C. elegans.
“I returned to a field that was starving for people to do EM,” Dr. Hall recalls. “When I said I was open for business, researchers from all over started sending me C. elegans mutants for imaging.”
Dr. Emmons would come to rely on Dr. Hall’s know-how for his connectome studies. In addition, Dr. Hall provided a priceless resource: the WormAtlas, a trove of roundworm data that would eventually include all of Dr. Brenner’s original connectome maps and images (see “WormAtlas”).
Constructing the male connectome was possible, but also arduous. In 2012, after a dozen years of work, Dr. Emmons and his colleagues published in Science the wiring diagram of the part of the nervous system controlling mating in the male roundworm. Their findings revealed that male mating requires 144 neurons—nearly half the worm’s total number—and described the connections among those 144 neurons and 64 muscles, involving more than 8,000 synapses.
“Our work [on] the underlying function that governs mating behavior in the male roundworm is a step toward identifying how an animal controls seemingly complex movements,” Dr. Emmons said at the time. In addition to finding out how the neurons and muscles are connected, the researchers for the first time accurately measured the weights of those connections—in other words, they estimated the strength with which one neuron or muscle communicates with another.
Dr. Emmons’ contribution to the roundworm canon was awarded the 2013 Newcomb Cleveland Prize by the American Association for the Advancement of Science (AAAS), recognizing the most outstanding paper published in Science from June 2012 to May 2013.
“This one paper emerged as a tour de force from amongst many competitive entries,” Science editor-in-chief Marcia McNutt remarked at the time. “The robust model system will contribute significantly to our further understanding of the precise mapping between neuron activity and essential behaviors that ensure survival of the species.”
Still, there were those who wondered what all the fuss over connectomes was about. At a 2012 debate held at Columbia University just before the publication of the Science paper, New York University neuroscientist Anthony Movshon remarked that the connectome “is a sort of a bed on which we can build experiments—and many people have built many elegant experiments on that bed. But that connectome by itself has not explained anything.”
It would take further research by Dr. Emmons to convince doubters that mapping neural connections was worth the effort.
Although the AAAS award would have been a fitting capstone to his career, Dr. Emmons had more dots to connect. Using new electron micrographs and Dr. Brenner’s old ones, he and his team compiled complete wiring diagrams of the adult male and the hermaphrodite roundworm. They found all the synapses connecting neurons with one another, as well as the synapses connecting nerves to muscles and other tissues, such as the gut and skin, and the connections between the muscle cells—including estimates of the strength of those synapses.
The EM images were digitized and analyzed using the group’s homegrown software, which significantly facilitated the process. Yet it took seven more years of work before Dr. Emmons and his colleagues could complete the two connectomes and publish their maps in July 2019 in Nature. By diagramming both the male and female C. elegans nervous systems, the researchers were able to make the first comprehensive comparison of the wiring of the sexes.
“While the synaptic pathways in the two sexes are substantially similar, a number of the synapses differ in strength, providing a basis for understanding sex-specific behaviors,” Dr. Emmons says.
Since the roundworm nervous system contains many of the same molecules as the human nervous system, what we learn about the former can help us understand the latter. — Dr. Scott Emmons
The primary sex differences pertain to reproductive functions: for the hermaphrodite, it’s the vulval and uterine muscles and the motor neurons that control them; for the male, it’s the large number of additional neurons, sex muscles, and connections in the tail that generate the circuits for copulation. But beyond those reproductive differences, a surprising percentage—up to 30%—of synapses between neurons in central pathways shared by both sexes also appear to differ considerably in strength.
The Nature paper attracted a lot of attention in both the lay and scientific press, including major articles in The New York Times, The Washington Post, The Telegraph, The Guardian, and New Scientist. The findings mark “a major step forward in the attempt to understand how a brain’s function emerges from its form,” wrote University of Rochester neuroscientist Douglas S. Portman in a “News & Views” article accompanying Dr. Emmons’ paper in Nature.
“Depicted graphically, the new connectomes don’t obviously resemble artificial neural networks or the wiring schematics of simple electronic devices; they look more like the cobwebs that lurk at the back of the broom cupboard,” Dr. Portman added. “Although intriguing patterns can be identified, distinct circuits for specific behavioral responses are not readily apparent. As others have pointed out, the connectome is only a map of possibilities.”
But those very possibilities are what drive researchers in connectomics. “These connected networks serve as starting points for deciphering the neural control of C. elegans behavior,” Dr. Emmons says. “Since the roundworm nervous system contains many of the same molecules as the human nervous system, what we learn about the former can help us understand the latter.”
Dr. Emmons believes that the combined impact of his two major connectome papers—first in Science, then in Nature—has largely persuaded skeptics that plotting neural connections is worth the effort. “We not only mapped the connectome, we mapped the first natural neural network,” he says. “Using graph theory, we were able to separate the neurons and muscles into groups, and we could say that this group must be doing this and that one must be doing that—linking specific parts of the connectome to specific behaviors.”
Researchers have hypothesized that some neurological and psychiatric disorders, such as schizophrenia and autism, are “connectopathies”—that is, problems caused by “faulty wiring.”
“This hypothesis is strengthened by the finding that several mental disorders are associated with mutations in genes that are thought to determine connectivity,” Dr. Emmons says. “Connectomics has the potential to help us understand the basis of some mental illnesses,” possibly suggesting new treatment strategies. In concluding his Nature “News & Views” article, Dr. Portman wrote: “Once again, Brenner’s tiny worm, occupying its unique sweet spot between simplicity and complexity, finds itself on the front line of biology’s most challenging problems.”
Dr. Emmons is now studying how the roundworm’s wiring is encoded in the genome, a challenge as complex and laborious as his connectome studies. Meanwhile, other groups are attempting to draw wiring diagrams of more-complex model organisms, including the fruit fly and the mouse.
If these efforts are successful, connectomics will become even more relevant to the study of human biology. Whether neuroscientists will ever be able to map the human nervous system, with its 100 billion neurons and quadrillions of connections, is the stuff of far-off dreams.
“My imagination isn’t good enough to contemplate the implications of knowing the whole human connectome,” Dr. Emmons says. “It’s a profound thought, though. It could ultimately get to questions about the nature of consciousness.”