Sam (not his real name) was diagnosed with type 1 diabetes in his early teens. He struggled to follow the insulin regimen needed to control his blood-glucose level, even after experiencing diabetes-related complications that required hospitalizations. Finally, in his late 20s, Sam started handling self-management for his diabetes, but his effort came too late to prevent permanent disability.
All too many people with type 1 diabetes (T1D)—especially adolescents and young adults—fail to reach target blood-glucose levels. The elevated glucose forms toxic compounds that damage tissues, leading to potentially devastating consequences that occur in T1D as well as type 2 diabetes: diabetic coma, blindness, heart disease, kidney failure, nerve damage, and amputations.
“People talk about the type 2 diabetes epidemic—and it is certainly a major public health issue,” says Yaron Tomer, M.D., who is the chair of medicine at Einstein and Montefiore and a professor of medicine and of microbiology & immunology and the Anita and Jack Saltz Chair in Diabetes Research at Einstein. “But type 1 diabetes is much more common than people think. Since World War II, the frequency of type 1 has doubled every 20 years in almost every population group, and nobody knows why (See “Type 1 Diabetes By the Numbers,” below).


All too often, when left to their own devices, young adults with T1D neglect self-care or are unable to make it a priority. By early adulthood, they may already have started experiencing significant complications. “But if we support these patients and push them toward independence, we can change that trajectory,” Dr. Agarwal says.
SEAD is staffed by an endocrinologist (Dr. Agarwal); a nurse practitioner with expertise in type 1 diabetes and advanced technologies such as insulin pumps; a nurse specializing in social work and community care; and, notably, a psychologist—a rare combination in diabetes clinics. The team also offers peer-support groups and a yearly group retreat where participants can discuss T1D-related psychosocial issues and learn about technologies for managing their care, such as continuous glucose monitors and insulin pumps.
“We’re not going to improve everyone’s glycemic control,” Dr. Agarwal admits. “But engaging patients in care is the most important thing we can do. When they’re lost to the healthcare system is when things really go wrong.”
One of Dr. Agarwal’s goals is to create a model of care that can be adopted by providers around the nation, even those with limited resources. “Second, we need to demonstrate that our approach makes sense financially to healthcare providers and payers,” she says.
SEAD is a product of a larger project to reduce disparities in T1D care among young racial/ethnic minorities, funded by a five-year, $988,000 award from the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK). The project aims to identify modifiable factors that can reduce disparities and improve health outcomes, learn more about patient-provider relationships in these communities, and design and test interventions.
Sadly, SEAD came too late for Sam, who later in life became Dr. Agarwal’s patient. By his early 30s, he had gone blind from T1D. “It was heartbreaking when he told me he wished he’d had this program when he was younger,” Dr. Agarwal says. “Let’s hope we can prevent others from developing such devastating complications.”
We’re not going to improve everyone’s glycemic control. But engaging patients in care is the most important thing we can do.
— Dr. Shivani Agarwal
Clearly, we need to build a workforce that can deal with patients confronting the psychosocial aspects of diabetes care.
— Dr. Jeffrey Gonzalez
Dr. Gonzalez hopes to answer these and other questions in a study using smartphone apps and continuous glucose monitors (devices that are worn on the body that automatically and continuously measure blood-sugar levels).
“We usually ask patients to fill out paper-and-pencil questionnaires about their moods over a recent week and relate these to a single measure of A1C that may not overlap with that period,” he explains. “But memory is often imprecise and unreliable. With our app, we can query patients about their moods several times a day. Then we can correlate that info with data from continuous glucose monitors. This will give a better sense of how stress relates to blood glucose from moment to moment. We’ll also determine whether hyperglycemia and hypoglycemia are associated with changes in patients’ cognitive function, as has been suspected.
“We need to look beyond A1C,” he adds. “The long-term measure of blood sugar is important, but we also need to look at patients’ day-to-day experiences with diabetes, and at how those experiences can be improved.”
In the meantime, Dr. Gonzalez is also investigating how to make T1D care more patient-friendly and more accessible. Recent surveys show that most adults with T1D are seen not by endocrinologists but by primary care doctors, who are hard-pressed to give adequate time to patients with complex chronic diseases and may not be familiar with all the nuances of diabetes care.
“Smartphone apps are one approach; telehealth is another,” Dr. Gonzalez says. “Adding mental-health professionals to diabetes clinics, as we’ve done at the Fleischer Institute, would also be helpful. Unfortunately, it’s not financially rewarding to deliver mental-health care in this setting, at least in the short term. That’s part of the reason why it’s so hard to access. Part of my job as a researcher is to demonstrate to payers that this is an investment worth making.”
In sum, says Dr. Gonzalez, “clearly, we need to build a workforce that can deal with patients confronting the psychosocial aspects of diabetes care. But it’s also clear that we have a long way to go with that mission.”
T cells such as CD4+ T cells aren’t born knowing what to hunt for. Just as bloodhounds require a scent to track and find a missing person, T cells rely on peptides—bits of protein from invading bacteria, for example—to tell them what to attack. Those immunity-arousing peptides are called antigens.
T cells get their marching orders, in the form of peptide antigens, from another type of immune cell called antigen-presenting cells (APCs). Transferring a peptide from an APC to a T cell resembles spoon-feeding. The APC’s “spoon” is present on its surface as a tangle of proteins dictated by HLA genes. This spoon contains grooves, known as “pockets,” which accommodate peptides that APCs then “feed” to T cells.
However, some HLA gene variants create APC spoons whose pockets mistakenly bind the body’s own peptides. T-cell receptors recognize these “self” peptides—prompting the T cells to attack the body’s own cells and tissues and leading to autoimmune diseases.
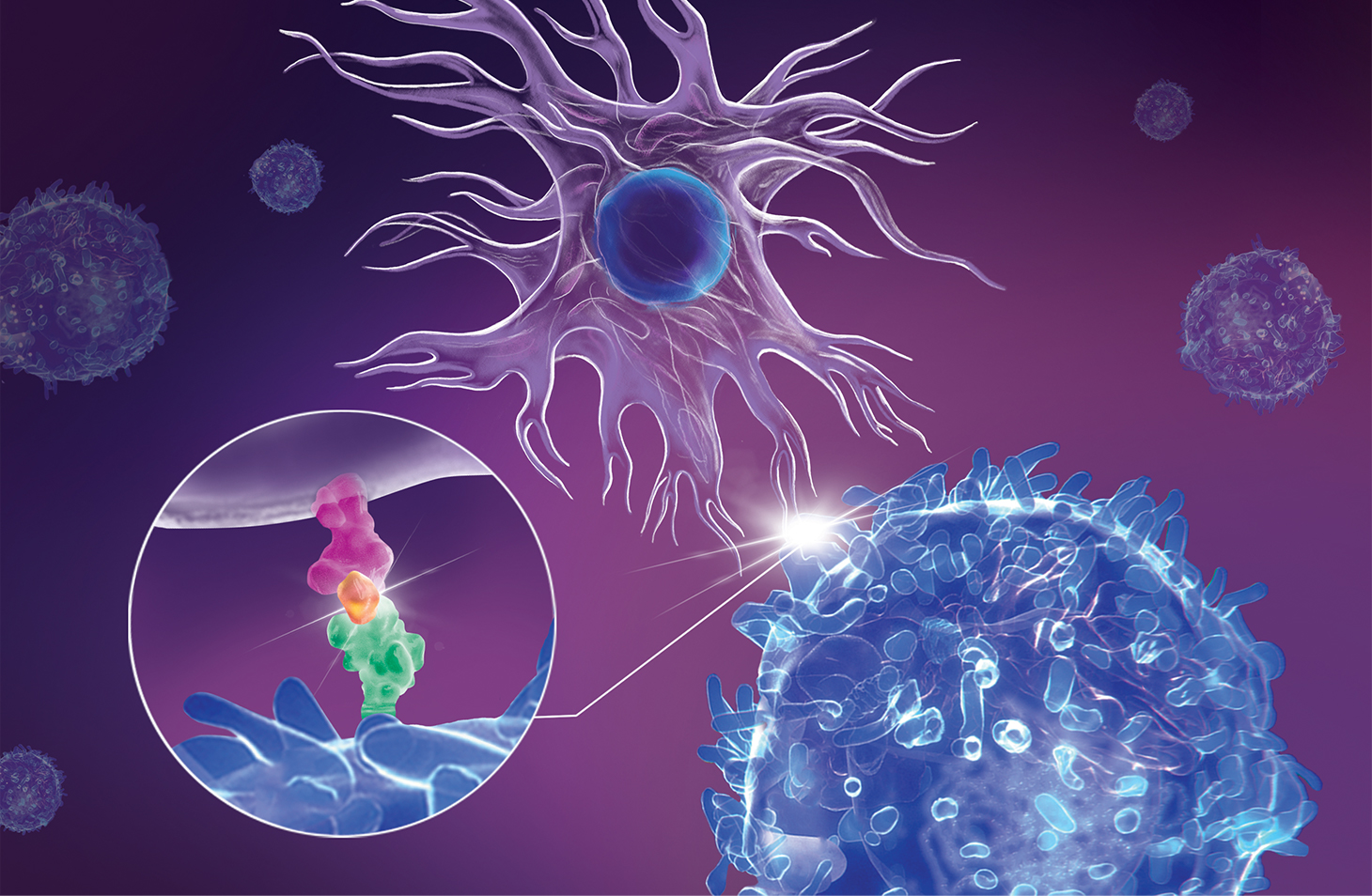 This illustration of an immunological synapse shows an antigen- presenting cell or APC (upper cell) interacting with a CD4+ T cell (lower cell). In the circular close-up of the synapse, an HLA protein (pink) on the APC’s surface is displaying a peptide (orange) that is being bound by a T-cell receptor (green). This interaction activates the T cell to attack cells containing that peptide. In type 1 diabetes, the display of peptides belonging to insulin-producing cells in the pancreas “miseducates” T cells to attack and destroy those pancreatic cells.
This illustration of an immunological synapse shows an antigen- presenting cell or APC (upper cell) interacting with a CD4+ T cell (lower cell). In the circular close-up of the synapse, an HLA protein (pink) on the APC’s surface is displaying a peptide (orange) that is being bound by a T-cell receptor (green). This interaction activates the T cell to attack cells containing that peptide. In type 1 diabetes, the display of peptides belonging to insulin-producing cells in the pancreas “miseducates” T cells to attack and destroy those pancreatic cells.
Most T1D cases occur when APCs bind insulin peptides, causing T cells to attack insulin-rich beta cells of the pancreas. To halt T1D, Dr. Tomer and his colleagues in the Einstein–Mount Sinai Diabetes Research Center are pursuing a novel strategy: Find peptide drugs to block the binding pockets of APCs susceptible to causing T1D.
Using computer modeling to screen thousands of potential compounds, they found one highly promising pocket-blocking candidate that they are now modifying to boost its effectiveness. It’s called a retro-inverso peptide.
“We made a mirror image of the original peptide and then reversed its amino acid sequence,” Dr. Tomer explains. “This configuration stabilizes the peptide so it sits securely within the APC’s pocket.” The experimental peptide has shown encouraging results in inactivating APCs in a T1D mouse model and in cells taken from patients with T1D.

We envision that this would be a personalized medicine for T1D.
—Dr. Teresa DiLorenzo
Early in the COVID-19 pandemic, doctors at Montefiore observed that about 40% of people hospitalized with the disease also had type 1 or type 2 diabetes. It was a striking observation but not a total surprise. People with diabetes are no more susceptible than others to viral infections, but they are more vulnerable to serious complications once infections occur. Factor in the high prevalence of diabetes in the Bronx, and the 40% figure makes sense.
What didn’t make sense were other aspects of the coronavirus-diabetes connection. Some patients with diabetes had well-controlled blood-glucose levels pre-COVID-19, but those levels became dangerously high and fluctuated wildly following infection.
Even stranger, COVID-19 may actually cause diabetes. Patients with no history of diabetes were showing up on the COVID-19 wards with ketoacidosis, a potentially deadly condition usually associated with type 1 diabetes (T1D). Ketoacidosis (the buildup of acidic substances called ketones) occurs when cells lack sufficient glucose for energy and burn fat instead.
“There are many interesting things about this virus—some more interesting than we’d like,” says Jill Crandall, M.D., professor of medicine, the Jacob A. and Jeanne E. Barkey Chair in Medicine, and chief of the division of endocrinology at Einstein and Montefiore.
As yet, clinicians have no definitive guide for caring for COVID-19 patients who have diabetes, so they’re learning by doing—and by sharing. Einstein and Montefiore’s endocrinologists are now contributing to a nationwide population health–surveillance study of individuals with T1D who contract COVID-19.
“We’re focusing this surveillance effort on type 1 diabetes, but we’re also interested in the effects of COVID-19 on type 2, the more-common form,” Dr. Crandall adds. She notes that patients will also be monitored after they return home, to see if COVID-19 has caused long-term changes to their diabetes.
Meanwhile, Dr. Shivani Agarwal (introduced earlier in article) is coordinating two pilot projects aimed at improving care for people with diabetes who develop COVID-19.
One project is assessing how well continuous glucose monitors (tiny devices that automatically measure glucose levels) will work on hospitalized patients.
“The monitors are approved for outpatients but not for the inpatient setting, where finger sticks are used,” Dr. Agarwal says. “But with nurses so busy and to reduce use of personal protective equipment and exposure to COVID-19, it would be better to automate this process and not use up protective gear just to take a glucose measurement. However, several factors could affect the accuracy of these monitors in the inpatient setting, and so we need to rigorously test this approach.”
A second project is evaluating whether subcutaneous insulin injections can replace intravenous insulin drips for managing patients with diabetic ketoacidosis.
“Insulin drips allow for fine-tuning of insulin to control blood sugar but are labor intensive since a lot of monitoring is needed, including hourly finger sticks,” Dr. Agarwal says. “In this context, periodic insulin injections may be the better alternative, especially because of COVID-19. Our number one priority is to maintain patient safety and optimize clinical outcomes, but we also need to reduce the burden on our nursing staff. We hope this approach will achieve both goals.”