The names “Verma and Steidl” don’t roll off the tongue the way “Brady and Belichick” and “Bert and Ernie” do. But it would be hard to imagine a more productive research duo.
That’s because over the past decade, longtime Einstein colleagues Amit Verma, M.B.B.S., and Ulrich Steidl, M.D., Ph.D., have coauthored several dozen peer-reviewed papers, adding immensely to the understanding of two closely intertwined diseases: myelodysplastic syndromes (MDS) and acute myeloid leukemia (AML). (See “The ABCS of MDS and AML,” below.)
Drs. Verma and Steidl were among the first researchers to demonstrate that MDS, a common precursor of AML, arises from wayward bone-marrow stem cells; the pair’s findings have raised hopes for treating or preventing both diseases.
The researchers began their journeys to Einstein from vastly different parts of the world. Dr. Verma studied medicine at the All-India Institute of Medical Sciences in New Delhi and then relocated to the United States for postgraduate training at the University of Illinois at Chicago Medical School, ultimately focusing his research and clinical practice on MDS. Around the same time, Dr. Steidl studied medicine at the University of Heidelberg in Germany and then cell and tumor biology at the German Cancer Research Center in Heidelberg before pursuing postdoctoral studies at Harvard Medical School, with a focus on AML.
Dr. Verma arrived at Einstein in 2007, followed by Dr. Steidl the next year. “We met right after I joined the faculty and discovered very quickly that we had complementary skills,” says Dr. Steidl, who is now a professor of cell biology and of medicine, the Diane and Arthur B. Belfer Faculty Scholar in Cancer Research at Einstein, and the associate chair for translational research in oncology at Montefiore. “My work is more lab-based; his is more translational, with a clinical component. I was looking for a partner who could help bring my laboratory findings to the clinic.”
In Dr. Steidl, Dr. Verma found someone with expertise in cell sorting, cell identification, and animal models of myeloid diseases (i.e., those pertaining to bone marrow). “He provided a perfect counterpart to my lab’s expertise in deciphering cell-signaling and epigenetic pathways and studies of clinical samples,” says Dr. Verma, professor of medicine and of developmental and molecular biology at Einstein and director of hemato-oncology at Montefiore. The pair, who are both members of the National Cancer Institute-designated Albert Einstein Cancer Center, soon began to collaborate on MDS and AML research.
MDS, a cancer of the bone marrow, occurs when blood-forming cells called “blasts” become dysplastic (that is, they develop abnormally) and multiply rapidly. Abnormal blasts churn out defective blood cells and leave people with too few normal ones. Different types of MDS can decrease different blood cells—most commonly red cells, resulting in anemia.
MDS, a cancer of the bone marrow, occurs when blood-forming cells called “blasts” become dysplastic (that is, they develop abnormally) and multiply rapidly.
The incidence of MDS in the United States is unclear, with estimates ranging from 10,000 to 40,000 new cases annually. The only cure is a bone-marrow transplant—a therapy not easily tolerated and therefore reserved for the youngest, most resilient patients. Most people diagnosed with MDS, however, are elderly. Deaths result from bleeding, infection due to low blood-cell counts—and from MDS progressing to AML, which occurs in about one-third of MDS patients. (This is why MDS was once described as a “premalignant” condition but is now generally regarded as a form of cancer.)
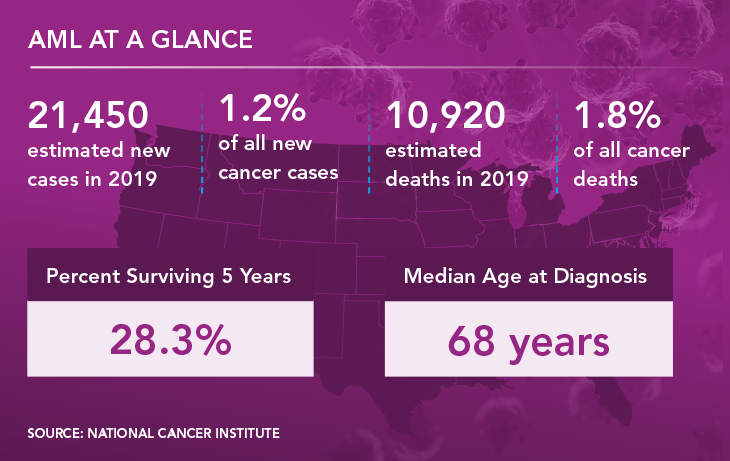
AML is an aggressive and usually fatal blood cancer that begins in the bone marrow. It afflicts about 21,000 Americans each year and is diagnosed when massive numbers of defective blast cells are detected in the bone marrow and blood. As is true for MDS, AML usually can be cured only with a bone-marrow transplant and mainly affects older people. Just 28% of AML patients survive for five or more years after diagnosis.
Targeting the blasts is like mowing dandelions in your lawn. They’ll always regrow unless you get to the root of the weed.
— Dr. Ulrich Steidl
By 2008, when Drs. Verma and Steidl began collaborating, studies had shown that AML arises from defective hematopoietic (blood-forming) stem cells, the bone-marrow cells that develop into blasts and, ultimately, all the blood’s cell types. By contrast, cases of MDS were thought to begin later in blood-cell development, when blood-progenitor cells became dysplastic and could no longer produce fully mature red cells, white cells, and platelets, instead producing an excess of malignant blast cells. These defective blasts were the target of MDS treatments.
“An MDS therapy was viewed as a success if it significantly reduced the blast count,” Dr. Verma says. “This approach was effective at improving symptoms in many patients, even putting some into remission. But invariably, the disease would come back.” Targeting the blasts, says Dr. Steidl, “is like mowing dandelions in your lawn. That will get rid of them, but they’ll always regrow unless you get to the root of the weed.”
So what is the actual cause of MDS? Relying on animal evidence, Drs. Verma and Steidl, along with a few other scientists, suspected that MDS—like AML—originated from defective hematopoietic stem cells.
“It was hard to get our MDS research funded,” Dr. Steidl adds. “Grant reviewers would comment: ‘The problem in MDS is clearly the blasts and dysplastic cells. Why waste your time studying these tiny, barely detectable populations of hematopoietic stem cells?’”
But the Einstein researchers persisted. In a seminal study published in 2012 in the journal Blood, they analyzed bone-marrow stem cells and their slightly more differentiated progeny (progenitor cells) from 17 patients with various types of MDS and 16 healthy controls. Genome-wide analysis revealed widespread genetic and epigenetic alterations in stem cells from MDS patients, but not in stem cells taken from healthy controls. Those same gene alterations were also found in patients’ blasts, which evolve from stem cells.
“Equally important,” Dr. Verma says, “we found that abnormal stem cells persist in the bone marrow even after standard chemotherapy for MDS. So although the patient may be in remission, those stem cells resist chemotherapy and the disease will inevitably return. Our findings made it clear that we needed to wipe out the abnormal stem cells to improve cure rates.”
Our findings made it clear that we needed to wipe out the abnormal stem cells to improve cure rates.
— Dr. Amit Verma
Since publishing their initial paper, Drs. Verma and Steidl have sought ways to disable abnormal stem cells in patients with MDS and AML. Thus far they’ve found a half-dozen vulnerabilities in such stem cells, and experimental drugs targeting them are now in clinical trials.
A key recent contribution involves ALRN-6924, the first of a novel class of drugs called stapled peptides (small chains of amino acids with helical structures that are stabilized using hydrocarbon “staples”). Aileron Therapeutics developed ALRN-6924 to reactivate the p53 tumor-suppressor gene, which prevents impaired or cancerous cells from multiplying.
The p53 gene is inactivated in virtually all cancers and in the mutated stem cells that develop into AML, so reactivating p53 might be expected to stop tumor cells in their tracks. But so far, no stapled-peptide drug has advanced to clinical trials—until now.
In a novel approach, Aileron scientists designed ALRN-6924 to reactivate p53 indirectly by simultaneously inhibiting two proteins, MDMX and MDM2, that are overexpressed in cancer and act together to suppress key p53 functions. The company then turned to Drs. Verma and Steidl to test the drug’s effectiveness in preclinical models of AML and to learn more about its mechanism of action.
The Einstein researchers found that ALRN-6924 had a profound impact on AML, tripling the median survival time in an animal model of human AML (mice transplanted with human leukemia cells) from 50 to about 150 days.
“This is a very striking response,” Dr. Steidl says. “Most experimental drugs for leukemia achieve an increase in survival of only a few days in these preclinical models. More important, ALRN-6924 effectively cured about 40% of the treated mice, meaning they were disease-free more than one year after treatment—essentially a lifetime for a mouse.”
The researchers confirmed that ALRN-6924 targets both MDMX and MDM2, blocking their interaction with p53 in AML cells. That effect was seen in both immature and mature AML cells. Furthermore, those same molecular changes were observed in blood cells of an AML patient who was given the drug on a compassionate-use basis.
“This test was not designed to assess the efficacy of the drug in humans—that has to be done in a proper clinical trial,” Dr. Steidl says. “Our goal was to determine whether it can hit the desired target in human cells in a clinical setting, which it did in this individual.”
The ALRN-6924 findings, reported in Science Translational Medicine in 2018, have led to a phase 1/phase 2 clinical trial for patients with advanced AML or MDS, now underway at Montefiore and three other centers. Dr. Verma is leading Montefiore’s portion of the trial. He directs Montefiore’s MDS Center, which offers a variety of treatments and clinical trials for patients with the disease and is recognized as a national “center of excellence” by the MDS Foundation.
Earlier this year, Drs. Verma and Steidl tackled another vexing question: What causes one-third of MDS cases to progress to the usually fatal AML?
In research published in Nature Medicine in 2019, they and their colleagues carried out single-cell genomic sequencing of stem cells and blast cells from seven patients with MDS that progressed to AML.
“The old linear model—MDS blast cells accumulate increasing numbers of mutations, which ultimately lead to AML—didn’t hold up,” Dr. Steidl says. “Instead, we found that the progression from MDS to AML begins earlier, with a diverse spectrum of mutations in hematopoietic stem cells. A defective stem cell will evolve into MDS, but it is not those MDS cells that develop into AML. Instead, other aberrant stem cells—ones that arise from different defective stem-cell ‘subclones’—acquire additional mutations that ultimately lead to AML. So to prevent MDS from evolving into AML, we need to focus our efforts on detecting and targeting those early aberrant stem cells.” (See illustration above.)
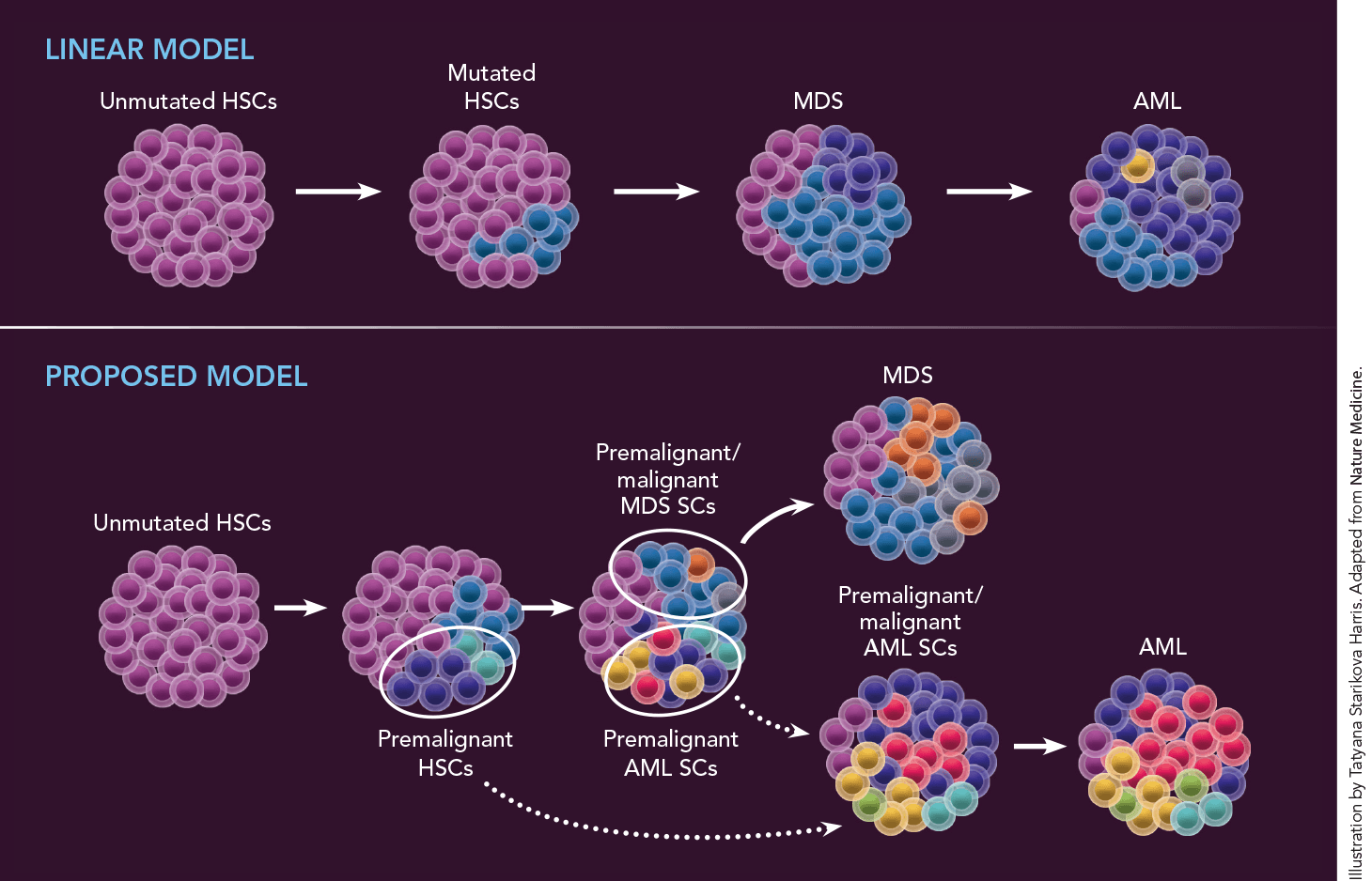 Why do one-third of MDS patients eventually develop AML? The standard linear model (at top) proposes that hematopoietic stem cells (HSCs) accumulate additional mutations that ultimately cause MDS to evolve into AML. But after conducting single-cell genomic sequencing of stem cells (SCs) from seven patients with MDS who later developed AML, Drs. Verma and Steidl created a model (above) positing that MDS and AML are actually separate diseases that evolve from different aberrant stem-cell subclones, i.e., cell groups that descend from a single mutated cell very early in disease development. MDS (which, for unknown reasons, often precedes AML) evolves from premalignant/malignant MDS stem cell subclones; AML independently evolves from aberrant stem-cell subclones that differ from those that led to MDS and that may have been present for many years before MDS or AML develops. Dotted lines indicate uncertainty regarding exactly when one stem-cell type progressed to another in some patients.
Why do one-third of MDS patients eventually develop AML? The standard linear model (at top) proposes that hematopoietic stem cells (HSCs) accumulate additional mutations that ultimately cause MDS to evolve into AML. But after conducting single-cell genomic sequencing of stem cells (SCs) from seven patients with MDS who later developed AML, Drs. Verma and Steidl created a model (above) positing that MDS and AML are actually separate diseases that evolve from different aberrant stem-cell subclones, i.e., cell groups that descend from a single mutated cell very early in disease development. MDS (which, for unknown reasons, often precedes AML) evolves from premalignant/malignant MDS stem cell subclones; AML independently evolves from aberrant stem-cell subclones that differ from those that led to MDS and that may have been present for many years before MDS or AML develops. Dotted lines indicate uncertainty regarding exactly when one stem-cell type progressed to another in some patients.
For the first time in their careers, Drs. Verma and Steidl are optimistic that better therapies for MDS and AML—ones that target stem cells—are close at hand. “The survival rates for MDS and AML have not improved for the last half-century, and nobody understood why,” Dr. Steidl says. “Now we know it’s because we weren’t focusing on the right cells. It makes me very hopeful.”
They expect to continue their partnership. “We’re very good friends,” says Dr. Verma about his colleague. “Uli’s a great guy, very sharp, with the ability to solve complex problems logically. Beyond that, we have built the research infrastructure for our work. Science is so much more complex these days. It’s tough for one lab to have mastery of every possible technique.”
Adds Dr. Steidl: “I don’t think we’ve ever had an argument about our research. We try to take our egos out of the equations as much as we can. I feel very fortunate to have such a great collaborator. It was luck that we connected and clicked.”
The myelodysplastic syndromes (MDS) are a diverse group of disorders in which mutated hematopoietic stem cells result in the formation of vast quantities of defective red blood cells, white blood cells, and platelets. In most cases, the cause of these
mutations is not known. People at elevated risk for developing MDS are smokers or have been exposed to cancer therapies (such as chemotherapy and radiation), toxic chemicals, or heavy metals—all of which can damage DNA.
Patients with MDS are typically treated with specialized chemotherapies to reduce the number of blood-forming cells called “blasts,” plus symptom management and supportive care, such as blood transfusions. Treatment puts some patients into remission, sometimes for years, but the disease almost always returns. Survival varies widely depending on the disease type; it ranges from nine months for those with very high-risk MDS to 8.8 years for those with very low-risk disease.
Although one-third of MDS patients eventually develop acute myeloid leukemia (AML), the majority of AML cases arise without being preceded by MDS. AML shares many symptoms with MDS, including fatigue, shortness of breath, pale skin, susceptibility to infections, and frequent nosebleeds.
Treatment for AML depends on the stage and subtype of the disease, but usually includes chemotherapy, symptom management, and palliative care. Survival is heavily age dependent: For people diagnosed before age 20, the five-year survival rate is 67%; for those 20 and older, it’s only 24%.