“There is always one moment in childhood when the door opens and lets the future in,” the author Graham Greene wrote. For Steve Almo, Ph.D., that moment occurred when he was a toddler. “I was sick in bed and my dad brought me a guppy to take my mind off how I was feeling,” he recalls. “I was totally fascinated and obsessed with that little fish.” He started collecting aquatic animals the way other boys collected baseball cards. By sixth grade he was delving into chemistry and physics through Isaac Asimov’s nonfiction science books. “I knew by then I wanted to be a scientist,” he says.
As luck would have it, young Steve’s North Miami Beach high school had a program that offered budding scientists the chance to do real science. He wound up in a lab at the University of Miami School of Medicine that was studying enzymes. The work included looking at X-ray crystal structures of enzymes, as determined by other laboratories, to understand how those enzymes influence human health and disease.
I didn’t just want to look at the images. I wanted to make them.
— Dr. Steve Almo
X-ray crystallography involves the painstaking art of purifying and crystallizing biologic materials and then bombarding them with X-rays to reveal their three-dimensional molecular architecture. “But I didn’t just want to look at the images,” he says. “I wanted to make them.”
Even then, it was clear that the images were more than pretty pictures. As Dr. Almo explained decades later in an Einstein-produced video:
“The shape of a protein provides a huge blueprint as to its function. Once you know the function of the protein, you understand how it operates under normal conditions … but [knowing their shape] also gives you the opportunity to modify the activity of these proteins for therapeutic purposes.”
It’s safe to say that Dr. Almo was the only kid in his high school class who dreamed of becoming a crystallographer. He would dedicate the next two decades of his life to realizing that dream. He studied biology as an undergrad at the Massachusetts Institute of Technology and earned a Ph.D. in biophysics at Harvard University, where he mastered crystallography techniques, with a focus on enzymes.
After a postdoctoral fellowship at the Johns Hopkins School of Medicine, he ventured from Baltimore to the Bronx in 1992 to join the Einstein faculty—despite his preference for the T-shirt-and-shorts weather of his Miami home. “The biochemistry department at Einstein was—and still is—spectacular, with people like Vern Schramm [Ph.D.] and John Blanchard [Ph.D.], who are focused on the basic mechanisms and underlying chemistry of biology,” he says.
Thanks to his amazing intellectual leaps, Steve went from the role of a structural biologist to a leader in understanding the regulation of immune function.
— Dr. Vern Schramm
His first years at Einstein saw Dr. Almo applying his skills to the study of the actin cytoskeleton—the diaphanous protein filaments that give cells their shape and control how they divide. He also oversaw the automation of protein production, supplying scientists at Einstein and the broader research community with high-quality materials for investigations.
Supported by consistent and generous grants from the National Institutes of Health (NIH) (see “Protein Structures, Enzyme Functions—and a Halt in Funding,” below), Dr. Almo might well have continued solely as a crystallographer if not for a serendipitous early-2000s meeting with Einstein colleague Stanley Nathenson, M.D., an eminent immunologist. Dr. Nathenson had helped discover the molecular and cellular mechanisms that initiate the immune response to pathogens and malignancies.
“I got on the elevator on the ground floor of the Forchheimer Building, and there was Stan,” Dr. Almo recalls. “We had a brief chat as we rode up, and when he got out on the fourth floor, I said, ‘I think we could work together on some things.’ Our collaboration lasted more than a decade and was the beginning of my second career, as an immunologist.” The pair would go on to co-author more than three dozen peer-reviewed papers.
“Thanks to his amazing intellectual leaps, Steve went from the role of a structural biologist to a leader in understanding the regulation of immune function,” says Dr. Schramm, professor of biochemistry and the Ruth Merns Chair in Biochemistry and a pioneer in enzymology and drug design.
In 2010, Dr. Almo was awarded two separate five-year National Institutes of Health (NIH) grants totaling some $40 million, which kept him busy on several fronts.
For the first of those grants, he co-led a new enzyme-function initiative that brought together a diverse group of scientists nationwide to better understand the breadth of enzymatic and metabolic activities that exist in nature.
Under the second and larger of the grants, he directed a multicenter study of the structure and function of thousands of biomedically important proteins—part of the NIH’s Protein Structure Initiative (PSI), a federal, university, and industry effort to reduce the costs and time needed to determine a protein’s three-dimensional structure from its DNA sequence. “Using this knowledge, we can begin to learn how proteins can be modified to create new, highly targeted therapies for disease,” Dr. Almo said then.
At about that same time, Dr. Almo was also partnering with another PSI group, the Immune Function Network, led by Dr. Stanley Nathenson. This consortium of immunologists, geneticists, computational biochemists, and high-throughput structural biologists was studying cell-surface molecules that control the immune response, as well as substances that major pathogens secrete to evade the immune system or interfere with host signaling pathways.
In 2013, just three years after creating the PSI, the NIH abruptly announced that it would stop funding the initiative. Some people in the structural-biology community applauded the move, contending that smaller, investigator-initiated studies were more cost-effective and productive. But Dr. Almo and others questioned the NIH decision, arguing that PSI was just starting to pay off. “We’re now at the point where we can apply this protein-production infrastructure to improving human health,” an online article on Labmanager.com quoted him as saying. “You couldn’t ask for a better set of projects with genuine relevance to human disease. But such projects can’t be performed by a single investigator—they absolutely require different institutions working collaboratively.”
By this time, Einstein had invested millions in automated tissue-culture robotics to make large quantities of essential proteins involved in immunity. Fortunately, that effort didn’t go to waste. Although PSI funding wasn’t renewed, Dr. Almo managed to continue much of the work he had started under the grant—some of which led directly to the creation of Cue Biopharma.
In one of those intellectual leaps, Dr. Almo capitalized on his unusual mix of expertise—in structural biology, protein science, and immunology—to improve cancer immunotherapy.
Immunotherapy for cancer debuted in 2011, when the U.S. Food and Drug Administration approved ipilimumab (Yervoy) to treat metastatic melanoma. Ipilimumab is a monoclonal antibody that stimulates the immune system’s T cells to attack tumors. The drug was a genuine breakthrough against a cancer that had almost always proved fatal.
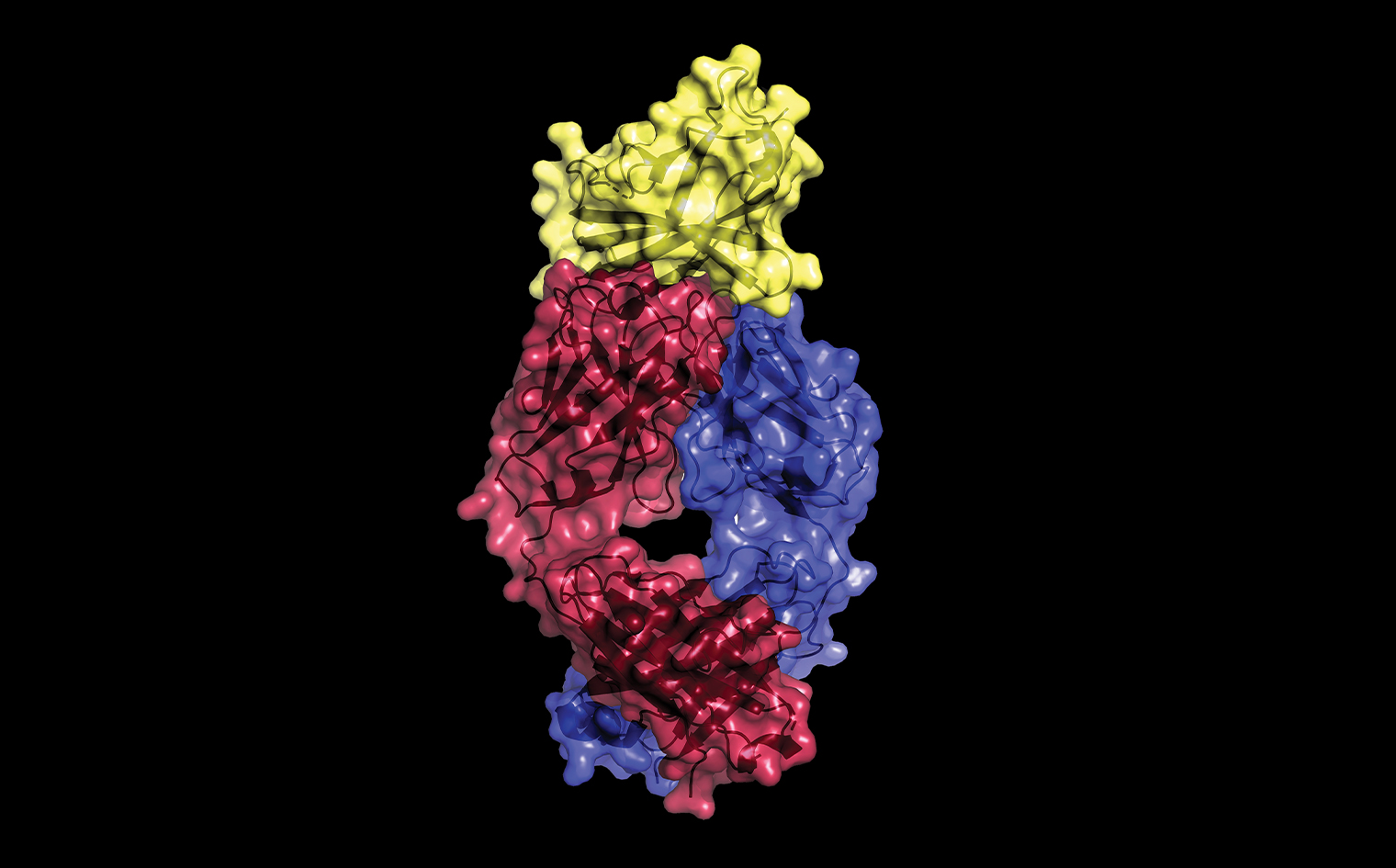 This X-ray crystallography image, made by Dr. Almo and colleagues, reveals the molecular basis for the therapeutic action of Yervoy, the monoclonal antibody and pioneering immunotherapy drug for treating metastatic melanoma and other cancers. The image shows how Yervoy's two protein chains (magenta and blue) interact with CTLA-4 (yellow), a receptor on the surface of T cells. Cancer-cell proteins thwart immune-system attack by stimulating CTLA-4, causing T cells to become inactive. By overlapping a portion of CTLA-4’s surface, Yervoy preserves T-cell activity by blocking cancer-cell proteins from binding to CTLA-4. The findings were reported in 2017 in PNAS.
This X-ray crystallography image, made by Dr. Almo and colleagues, reveals the molecular basis for the therapeutic action of Yervoy, the monoclonal antibody and pioneering immunotherapy drug for treating metastatic melanoma and other cancers. The image shows how Yervoy's two protein chains (magenta and blue) interact with CTLA-4 (yellow), a receptor on the surface of T cells. Cancer-cell proteins thwart immune-system attack by stimulating CTLA-4, causing T cells to become inactive. By overlapping a portion of CTLA-4’s surface, Yervoy preserves T-cell activity by blocking cancer-cell proteins from binding to CTLA-4. The findings were reported in 2017 in PNAS.
Unfortunately, not all melanoma patients responded to the drug. Only a minority were cured, and many patients suffered serious, even fatal, side effects. A major problem with Yervoy—and other first-generation immunotherapies such as Keytruda and Opdivo—was their unfocused approach: by stimulating all T cells rather than just those that potentially could mobilize against a tumor, the drugs unleashed uncontrolled and overzealous immune responses.
Dr. Almo, who is also co-leader of the cancer therapeutics program at the Albert Einstein Cancer Center, envisioned an entirely new type of immunotherapy, one that targeted cancer by activating a select group of T cells rather than all of them—a strategy that should greatly reduce the devastating side effects that can accompany the use of standard cancer immunotherapies.
The first step would be to identify, by their unique cell-surface proteins, those T cells relevant to a particular type of cancer. Next, researchers would synthesize molecules consisting of two fused protein “arms”: one arm specially designed to “dock” the fusion protein onto the desired T cells, and the other arm tailor-made to activate the T cells by stimulating particular receptors.
Dr. Almo dubbed this novel class of synthetic fusion proteins “synTac,” short for “synapse for T-cell activation.” “It’s like mailing a letter,” he explains, “with an address that delivers it to receptors on T cells relevant to our disease, plus a message telling those T cells what to do.” (See comic strip above.)
A key asset of the synTac platform is how easily it can target any of the many diseases—including autoimmune diseases—in which T cells play a role. For a particular disease, researchers can engage the relevant T cells simply by tweaking the synTac mailing address (the first arm of the fusion protein). This flexibility allows scientists to use synTac proteins against autoimmune diseases by programming the fusion proteins’ second arms to suppress, rather than stimulate, T-cell activity that injures the body.
By 2016, synTac had matured from a far-out idea to a potential therapy for human diseases. Dr. Almo and his colleagues had synthesized a number of different synTac proteins and had shown that they worked in cells and, potentially, in animal models of human disease. The researchers also had evidence that synTac activation of cancer-relevant T cells greatly increased the cells’ number and potency. But the path ahead for the novel synTac platform was highly uncertain.
“We were standing on the precipice of the ‘Valley of Death,’” says Dr. Almo, using drug-development lingo for the gap between invention and commercial application, where many advances perish for lack of funding. “We received vital NIH support for our initial lab work on synTac, but more funding is always needed to take promising drug candidates to the next level.”
Fortunately, help would come from Einstein’s office of biotechnology and business development, which interested a group of venture capitalists in the synTac technology. In 2017, Dr. Almo teamed with those investors to form Cambridge, Massachusetts–based Cue Biopharma, Inc., which licensed the synTac technology from Einstein and assumed the daunting tasks of developing synTac compounds into experimental drugs, evaluating those drugs in clinical trials, and gaining marketing approval for them.
In 2019, Cue Biopharma launched phase 1 trials of a synTac fusion protein called CUE-101, aimed at treating head and neck cancers caused by human papillomavirus. Several other Cue Biopharma synTac compounds are in various stages of preclinical development for treating cancers, autoimmune diseases, and infectious diseases.
Steve is one of Einstein’s most creative scientists. … So I was thrilled by the NIH grant enabling us to study his synTac platform for treating or perhaps even preventing type 1 diabetes.
— Dr. Teresa DiLorenzo
Dr. Almo is also partnering with Einstein colleagues to pursue synTac’s potential against other important diseases:
Melanoma and pancreatic cancer. In 2016, the NIH awarded Dr. Almo and Chandan Guha, M.B.B.S., Ph.D., a five-year, $2.6 million grant to develop synTac fusion proteins and evaluate their effectiveness in mouse models of melanoma and pancreatic cancer.
HIV and other viruses. Dr. Almo and Harris Goldstein, M.D., are developing synTac proteins that are programmed to stimulate the immune system’s CD8+ (“killer”) T cells to attack HIV-infected T cells. The research is supported by a five-year, $4.2 million grant awarded by the NIH in 2019. (The proteins are being tested in mice that have “humanized” immune systems and can therefore be infected with HIV.)
The scientists have also designed synTacs to stimulate killer T cells known to attack cells infected with cytomegalovirus, a common type of herpes virus that can infect and kill immunosuppressed patients. They have similar plans to use synTacs to bolster T cells that attack cells infected with Epstein-Barr virus, which causes mononucleosis and can also kill immunosuppressed patients.
Type 1 diabetes (T1D). This autoimmune disease occurs when T cells—normally defenders against cancers and disease-causing microbes—instead attack insulin-producing beta cells of the pancreas, leaving the body unable to control blood-glucose levels. Among the T cells implicated in T1D are CD8+ T cells.
Dr. Almo and Teresa DiLorenzo, Ph.D., are studying whether synTac proteins can intercept aberrant CD8+ T cells before they can reach and damage the pancreas. Their collaboration is funded by a four-year, $3.5 million NIH grant awarded in 2018. Dr. DiLorenzo is a professor of microbiology & immunology and of medicine and the Diane Belfer, Cypres and Endelson Families Faculty Scholar in Diabetes Research. She has devoted her career to T1D research.
“Steve is one of Einstein’s most creative scientists and is known for being a generous collaborator,” says Dr. DiLorenzo. “So I was thrilled by the NIH grant enabling us to study his synTac platform for treating or perhaps even preventing T1D. Our laboratory research shows that the synTac proteins Steve has designed can specifically inhibit T cells that attack beta cells, while leaving other T cells largely unaffected. We’ll soon begin in vivo studies of those synTac proteins using a mouse model of T1D.”
Adding to his responsibilities, in 2015 Dr. Almo assumed the chair of the department of biochemistry. “Steve is steering one of Einstein’s founding academic and research departments to new heights of excellence and innovation,” says Dr. Schramm, his immediate predecessor as department chair. “He has an uncanny ability to see the future of research programs and to make the science happen.”
Even after his promising foray into immunotherapy, Dr. Almo still defines himself as a structural biologist. “Everything we do starts with basic mechanisms, with understanding how things work at a detailed atomic and molecular level,” he says. “I try to foster this approach in the department of biochemistry, which I like to call the department of mechanism.” (See “An Ever-Expanding Portfolio,” below.)
Dr. Almo advocates “science for science’s sake,” a throwback to the time when researchers had more leeway to follow their intuition. “It’s great to have a focus on clinical applications,” he says. “But more often than not, the novel approaches come from unexpected places. Give creative, driven people the resources to follow their imagination, and something good will happen.”
Dr. Almo managed to keep his lab going during the pandemic, working remotely from his Westchester County home along with his wife, Anne Bresnick, Ph.D. (also a professor of biochemistry and the associate dean for postdoctoral affairs at Einstein), and their twin, college-bound daughters. Neither daughter has yet expressed interest in a science career. “Listening to Mom and Dad talk science at the dinner table all the time might have driven them away,” he says with a laugh.
While waiting for the pandemic to subside, Dr. Almo was able to indulge his passions for cooking and listening to jazz. “If I had my wish,” he says, “I would have been a jazz guitarist as opposed to a crystallographer, but I just had no talent.” Most would agree that music’s loss is science’s gain.
What’s next for Dr. Almo is hard to predict. “We’ve got so many things going on,” he says. “I’m really just trying to keep all of them afloat. But I’m always waiting for the next elevator ride.”

SynTac fusion proteins are just one part of Dr. Almo’s research, which has led to some 300 publications. Among his recent important findings: